One of the following compounds has an intense yellow fluorescence when irradiated with UV light. Which one
Question:
One of the following compounds has an intense yellow fluorescence when irradiated with UV light. Which one do you think it is, and why?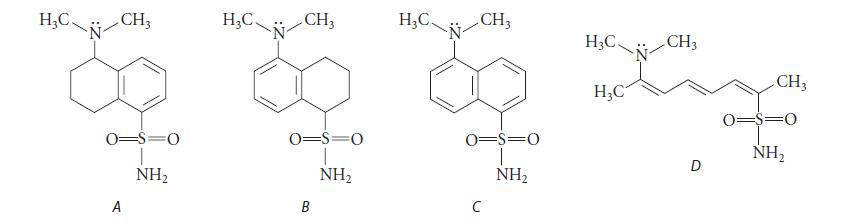
Transcribed Image Text:
H₂C. CH3 0=S=O A NH₂ H₂C. N CH₂ 0=S=0 NH₂ B H₂C. CH3 0=S=0 NH₂ C H3C. H₂C CH3 D CH3 0=$=0 NH₂
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
Based on the chemical structures of the four compounds shown in the image I think the compound that ...View the full answer

Answered By

PALASH JHANWAR
I am a Chartered Accountant with AIR 45 in CA - IPCC. I am a Merit Holder ( B.Com ). The following is my educational details.
PLEASE ACCESS MY RESUME FROM THE FOLLOWING LINK: https://drive.google.com/file/d/1hYR1uch-ff6MRC_cDB07K6VqY9kQ3SFL/view?usp=sharing
3.80+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Which one of the following compounds should have a * UV absorption at the greater max when the compound is dissolved in NaOH solution? Explain.
-
Refer to the Professional Judgment in Context feature at the beginning of the chapter. Additional details on PFG and Wasendorf are presented below. On July 14, 2012, Russell Wasendorf, Sr. attempted...
-
When a company has a winning product, it has it made. Or does it? Subaru is a winning company (one of the few automotive companies to sustain growth and profits in hard economic times) with various...
-
True or False? Azure storage is used by both Infrastructure as a Service ( ( laaS ) ) virtual machines, and Platform as a Service ( ( PaaS ) ) cloud services. True False
-
Why may net cash flow from operating activities on the cash flow statement be different from the amount of net income reported on the income statement?
-
According to Verto Analytics, Inc., 53% of 30- to-34-year-olds in the United States own tablets. (Data extracted from "The Impact of Income and Age on Device Ownership," bit.ly/1yR21gL.) Using the...
-
Consider an investor with an infinite horizon in a market with a constant risk-free return and a single risky asset with returns Rt = 1 e+ t for a sequence of independent standard normals t and a...
-
Roadster Company (RC) designs and produces automotive parts. In 2017, actual variable manufacturing overhead is $280,000. RC's simple costing system allocates variable manufacturing overhead to its...
-
Identify whether a debit or credit results in the indicated change for each of the following accounts
-
What products are formed in the DielsAlder reactions of the following dienes and dienophiles? (a) (b) and HCO-C. and HC=C COCH3 COCH3
-
Refer to Fig. 15.11. At what pH does the fluorescence of fluorescein have the greater quantum yield, pH = 9 or pH = 5? How do you know? relative absorbance 300 488 nm pH=9 pH = 5 400 wavelength, nm...
-
The following misstatements are sometimes found in the revenue account balances: 1. Cash amounts received from collections of accounts receivable in the subsequent period are recorded as current...
-
21. How a degradation process is modeled? 22.Give the homogenity property in Linear Operator 23. Give the relation for degradation model for continuous function 24.which is called the superposition...
-
28. Define Gray-level interpolation 29. What is meant by Noise probability density function? 30. Why the restoration is called as unconstrained restoration? 31. Which is the most frequent method to...
-
34. Give the relation for guassian noise 35. Give the relation for rayleigh noise 36. Give the relation for Gamma noise 37. Give the relation for Exponential noise 38. Give the relation for Uniform...
-
41. What is pseudo inverse filter? 42. What is meant by least mean square filter? 43. Give the difference between Enhancement and Restoration PART-B 1. Discuss different mean filters
-
1.Discuss different mean filters 2. Draw the degradation model and explain. 3.Write short notes on Median Filters
-
Use the sketch of s = f(t) on Figure 1 to approximate each of the following. (a) f'(2) (b) f'(6) (c) Vavg on [3, 7] (d) d/dt f(t2) at t = 2 (e) d/dt [f2(t)] at t = 2 (f) d/dt (f(f(t))) at t= 2
-
On average there are four traffic accidents in a city during one hour of rush-hour traffic. Use the Poisson distribution to calculate the probability that in one such hour there arc (a) No accidents...
-
The following compounds all show a single line in their 1H NMR spectra. List them in expected order of increasing chemical shift: CH4, CH2C12, cyclohexane, CH3COCH3, H2C = CH2, benzene
-
Predict the splitting pattern for each kind of hydrogen in the following molecules: (a) (CH3)3CH (b) CH3CH2CO2CH3 (c) trans-2-Butene
-
Predict the splitting pattern for each kind of hydrogen in isopropyl propanoate, CH3CH2CO2CH(CH3)2.
-
En prenant un exemple de votre choix, montrer comment on value un swap de taux de change.
-
How much would you need to invest today in order to receive: a. $10,000 in 5 years at 11%? b. $11,000 in 12 years at 8%? c. $12,000 each year for 10 years at 8%? d. $12,000 at the beginning of each...
-
A company that manufactures pulse Doppler insertion flow meters uses the Straight Line method for book depreciation purposes. Newly acquired equipment has a first cost of $190,000 with a 3-year life...

Study smarter with the SolutionInn App


