Refer to Fig. 15.11. At what pH does the fluorescence of fluorescein have the greater quantum yield,
Question:
Refer to Fig. 15.11. At what pH does the fluorescence of fluorescein have the greater quantum yield, pH = 9 or pH = 5? How do you know?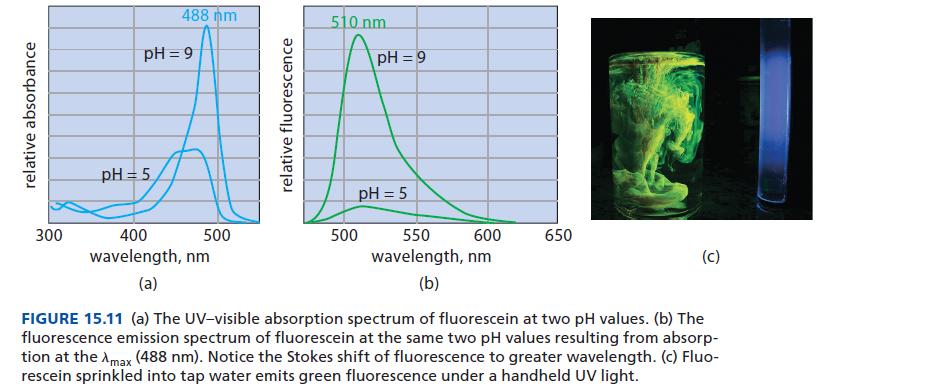
Transcribed Image Text:
relative absorbance 300 488 nm pH=9 pH = 5 400 wavelength, nm (a) 500 relative fluorescence 510 nm pH=9 pH = 5 500 600 550 wavelength, nm (b) 650 (C) FIGURE 15.11 (a) The UV-visible absorption spectrum of fluorescein at two pH values. (b) The fluorescence emission spectrum of fluorescein at the same two pH values resulting from absorp- tion at the Amax (488 nm). Notice the Stokes shift of fluorescence to greater wavelength. (c) Fluo- rescein sprinkled into tap water emits green fluorescence under a handheld UV light.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
The figure presented shows the UVvisible absorption spectrum and the fluorescence emission spectrum ...View the full answer

Answered By

Nyron Beeput
I am an active educator and professional tutor with substantial experience in Biology and General Science. The past two years I have been tutoring online intensively with high school and college students. I have been teaching for four years and this experience has helped me to hone skills such as patience, dedication and flexibility. I work at the pace of my students and ensure that they understand.
My method of using real life examples that my students can relate to has helped them grasp concepts more readily. I also help students learn how to apply their knowledge and they appreciate that very much.
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
You will need to save money to reach your financial goals. To save money, you will need a plan to show what money comes in and what money goes out. This is known as a budget. You may already have a...
-
You are interviewing a candidate for a position at a call center. You need someone polite, courteous, patient, and dependable. The candidate you are talking to seems nice. But how do you know who is...
-
The following question was asked of executives: How do you know when to cut off research? The answers given: Thats a good question, a very good question, and some people dont know when to cut it off....
-
A tape player has three operations: play, fast forward and fast play. Play and fast forward are activated using the play and fast forward button respectively. These operations can be cancelled using...
-
When are expenses recognized under accrual accounting?
-
The Society for Human Resource Management (SHRM) collaborated with Globoforce on a series of organizational surveys with the goal of identifying challenges that HR leaders face and what strategies...
-
In the model of Exercise 8.1, calculate the unique risk-neutral probability for any given horizon T < , and show that the risk-neutral probability of any path depends on t and the parameters Rf , k,...
-
A dated box of dates, of mass 5.00 kg, is sent sliding up a frictionless ramp at an angle of to the horizontal. Figure gives, as a function of time t, the component vx of the box's velocity along an...
-
The accounting rate of return is closest to 0.68% 8.33%. 12.00% 32.50% Click Save and Submit to save and submit. Click Save All Answers to save all answers
-
One of the following compounds has an intense yellow fluorescence when irradiated with UV light. Which one do you think it is, and why? HC. CH3 0=S=O A NH HC. N CH 0=S=0 NH B HC. CH3 0=S=0 NH C H3C....
-
Predict max for the UV absorption of each of the following compounds. (a) Et H T H H T H Et (b) H T H CH3
-
The manager of the Petroco Service Station wants to forecast the demand for unleaded gasoline next month so that the proper number of gallons can be ordered from the distributor. The owner has...
-
4. Write short notes on Wiener Filtering.
-
1.Explain Histogram processing
-
2. Explain Spatial Filtering ?
-
3. Explain the Geometric Transformations used in image restoration. 4.Describe homomorphic filtering
-
5.Explain the different Noise Distribution in detail. UNIT I V 1. What is segmentation? 2. Write the applications of segmentation. 3. What are the three types of discontinuity in digital image? 4....
-
An object moves on a horizontal coordinate line. Its directed distance s from the origin at the end of t seconds is s = t3 - 6t2 + 9t feet. (a) When is the object moving to the left? (b) What is its...
-
DEPARTMENT DATA EMPLOYEE DATA EmployeeNumber FirstName Mary Rosalie Richard George Alan 3 4 5 7 8 9 855555ES 12 13 14 15 16 17 Create the database tables in SQL or ACCESS: 18 19 20 PROJECT DATA Ken...
-
The acid-catalyzed dehydration of 1-methylcyclohexanol yields a mixture of two alkenes. How could you use 1H NMR to help you decide which waswhich? CH CH - 0* CH2
-
How could you use 1H NMR to distinguish between the following pairs ofisomers? (a) CH3CH=CHCH2CH3 and CH2 H H2H () CH20CH2CH and CH3OCH2CH2CH3 (c) CHC,H and CH3CH2CH3 (d) HCICH)H and CH3CH=CHCH3
-
Propose structures for compounds with the following formulas that show only one peak in their 1H NMR spectra: (a) C5H12 (b) C5H10 (c) C4H8O2
-
Your company produces a health magazine. Its sales data for 1 - year subscriptions are as follows: Year of Operation Subscriptions Sold % Expired at Year End 2 0 2 0 $ 3 0 0 , 0 0 0 5 2 0 2 1 $ 6 4 7...
-
Problem 3 - 2 0 ( Static ) Calculate profitability and liquidity measures LO 3 - 3 , 3 - 4 , 3 - 6 Presented here are the comparative balance sheets of Hames Incorporated at December 3 1 , 2 0 2 3...
-
3 Required information [The following information applies to the questions displayed below) John and Sandy Ferguson got married eight years ago and have a seven-year-old daughter. Samantha. In 2020,...

Study smarter with the SolutionInn App


