Predict max for the UV absorption of each of the following compounds. (a) Et H T
Question:
Predict λmax for the UV absorption of each of the following compounds.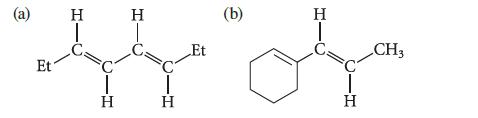
Transcribed Image Text:
(a) Et H T H H T H Et (b) H T H CH3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
Based on the image you sent here are my predictions for the max values for the UV absorption of each ...View the full answer

Answered By

Salmon ouma
I am a graduate of Maseno University, I graduated with a second class honors upper division in Business administration. I have assisted many students with their academic work during my years of tutoring. That has helped me build my experience as an academic writer. I am happy to tell you that many students have benefited from my work as a writer since my work is perfect, precise, and always submitted in due time. I am able to work under very minimal or no supervision at all and be able to beat deadlines.
I have high knowledge of essay writing skills. I am also well conversant with formatting styles such as Harvard, APA, MLA, and Chicago. All that combined with my knowledge in methods of data analysis such as regression analysis, hypothesis analysis, inductive approach, and deductive approach have enabled me to assist several college and university students across the world with their academic work such as essays, thesis writing, term paper, research project, and dissertation. I have managed to help students get their work done in good time due to my dedication to writing.
5.00+
4+ Reviews
16+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Predict the products when each of the following compounds is treated with NBS and irradiated with UV light: (a) (b) (c) (d)
-
Sunscreen contains compounds that absorb ultraviolet light. When sunscreen is applied to skin, it prevents ultraviolet light from reaching the skin. The graph that follows shows the absorbance of...
-
The UV spectrum of 1-phenylprop-2-en-1-ol shows an intense absorption at 220 nm and a weaker absorption at 258 nm. When this compound is treated with dilute sulfuric acid, it rearranges to an isomer...
-
If a company is very diversified, (a) it makes it easier to classify the company by industry. (b) it would not be necessary to provide any segmented information. (c) it can limit the usefulness of...
-
What type of transaction is a cash payment to creditors? How does this type of transaction affect the accounting equation?
-
An Australian Fair Work Commission survey of 1,134 employees across various industries gathered data on key drivers of job satisfaction. The most important aspect in determining employee satisfaction...
-
In the setting of Exercise 8.1, let P denote the physical probability and assume E Pt+1 +Dt+1 Pt = Rf . Suppose there is an infinite horizon. Show that there is no probability Q on the space of...
-
Following is partial information for the income statement of Audio Solutions Company under three different inventory costing methods, assuming the use of a periodic inventory system: Required: 1....
-
Burcham Corporation reported pretax book income of $532,500. Tax depreciation exceeded book depreciation by $425,000. In addition, the company received $257,000 of tax-exempt municipal bond interest....
-
Refer to Fig. 15.11. At what pH does the fluorescence of fluorescein have the greater quantum yield, pH = 9 or pH = 5? How do you know? relative absorbance 300 488 nm pH=9 pH = 5 400 wavelength, nm...
-
(a) From the extinction coefficient of isoprene (10,750 M 1 cm 1 ) and its observed absorbance at 222.5 nm (Fig. 15.5), calculate the concentration of isoprene in mol L 1 (assume a 1 cm light path)....
-
Well, okay, then, Grant conceded. Maybe I wont discontinue credit, but what can I do to take care of the problems Im having? What can Roy tell Grant?
-
6. What are the two properties used for establishing similarity of edge pixels? 7. What is edge? 8. Give the properties of the second derivative around an edge? 9. Define Gradient Operator? 10. What...
-
14. Define pattern. , 15. Define pattern class. 16. List the three pattern arrangements. 17. Give the decision-theoretic methods. 18. Define the training pattern and training set. 19. Define training...
-
1. Write short notes on image segmentation. 2. Write short notes on edge detection 3.Write Short notes on edge linking by local processing.
-
4. Write short notes on the applications of artificial neural networks in image processing.
-
What are the functions of a finance manager of a small firm?
-
An object is projected directly upward from the ground with an initial velocity of 128 feet per second. Its height s at the end of /seconds is s = 1281 - 16t2 feet. (a) When does it reach its maximum...
-
Why are stocks usually more risky than bonds?
-
How many 13C NMR absorptions would you expect for cis-1, 3-dimethyl- cyclohexane for trails-1, 3-dirnethylcyclohexane? Explain.
-
Assume that you have a compound with formula C 3 H 6 O. (a) How many double bonds arid/or rings does your compound contain? (b) Propose as many structures as you can that fit the molecular formula....
-
How could you use 1H and 13C NMR to help you distinguish among the following isomeric compounds of formulaC4H8? CH2-CH2 H2H CH CCH3HCH CH2-CH2 CHH2
-
Imagine you are an Investor in the Stock Market. Identify three companies in the Korean Stock Market (KOSPI) where you would like to invest. Explain your answer
-
Domino is 4 0 years old and is married out of community of property with the exclusion of the accrual system to Dolly ( 3 5 ) . They have one child, Domonique, who is 1 1 years old. Domino resigned...
-
YOU ARE CREATING AN INVESTMENT POLICY STATEMENT FOR JANE DOE General: 60 years old, 3 grown children that are living on their own and supporting themselves. She is in a very low tax rate so we don't...

Study smarter with the SolutionInn App


