Outline a preparation of each of the following compounds from aniline and any other reagents. (a) 2,4-dinitroaniline
Question:
Outline a preparation of each of the following compounds from aniline and any other reagents.
(a) 2,4-dinitroaniline
(b) Sulfathiazole, a sulfa drug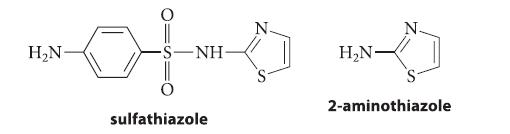
Transcribed Image Text:
H₂N- -NH- sulfathiazole H₂N- 2-aminothiazole
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (4 reviews)
a Begin with pnitroacetanilide prepared as shown in Study Pro...View the full answer

Answered By

JAPHETH KOGEI
Hi there. I'm here to assist you to score the highest marks on your assignments and homework. My areas of specialisation are:
Auditing, Financial Accounting, Macroeconomics, Monetary-economics, Business-administration, Advanced-accounting, Corporate Finance, Professional-accounting-ethics, Corporate governance, Financial-risk-analysis, Financial-budgeting, Corporate-social-responsibility, Statistics, Business management, logic, Critical thinking,
So, I look forward to helping you solve your academic problem.
I enjoy teaching and tutoring university and high school students. During my free time, I also read books on motivation, leadership, comedy, emotional intelligence, critical thinking, nature, human nature, innovation, persuasion, performance, negotiations, goals, power, time management, wealth, debates, sales, and finance. Additionally, I am a panellist on an FM radio program on Sunday mornings where we discuss current affairs.
I travel three times a year either to the USA, Europe and around Africa.
As a university student in the USA, I enjoyed interacting with people from different cultures and ethnic groups. Together with friends, we travelled widely in the USA and in Europe (UK, France, Denmark, Germany, Turkey, etc).
So, I look forward to tutoring you. I believe that it will be exciting to meet them.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Outline a Preparation of the following compounds from aniline and any other reagents. Sulfathiazole, a sulfa drug. H2N sulfathiazole
-
Outline a preparation of each of the following compounds from acetylene and any other reagents. (a) 1-hexene (b) 1-hexyne (c) trans-3-decene (d) (Z)-3-hexen-l-ol
-
Outline a preparation of sulfanilamide, a sulfa drug, from aniline and any other reagents. HN- 0=5 O -SNH, sulfanilamide
-
Which of the following is NOT a factor to be considered in determining a limited-life intangible assets useful life? The expected useful life of any related asset All of the other answers are correct...
-
Name and describe three areas of specialization for a public accountant.
-
Predict what will happen to stock prices after a monetary easing. Explain your prediction.
-
Discuss the factors to be taken into account when choosing an appropriate source of finance.
-
A blending tank that provides nearly perfect mixing is connected to a downstream unit by a Long transfer pipe. The blending tank operates dynamically like a first-order process. The mixing...
-
Oriole Information Technology Company has the following cost and net realizable value data at December 3 1 , 2 0 2 4 : \ table [ [ Inventory Categories,Cost,Net Realizable Value ] , [ Personal...
-
As shown in the following equation, when (R)-1-deuterio-1-butanamine is diazotized with nitrous acid in water, the alcohol product formed has the S configuration (D = 2 H). (a) Give the...
-
Provide a reaction mechanism for the reaction shown in Eq. 23.36. NH 5- + 3 Br Br NH Br Br + 3HBr (23.36)
-
Duncan Bostock is a sole proprietor in his first year of operations. The following table summarizes his taxable supplies for the current year: (a) When is Duncan required to register for GST/HST? (b)...
-
1. The KYM company wants to invest $ 523,000 pesos in the bank that guarantees a simple interest rate of 3.32% quarterly. If the company is considering the 8-month investment. What amount will you...
-
3. (5 points) The uncertainty principle limits our ability to determine simultaneously the position and momentum of a particle. (a) Why were classical physicists unaware of the limitations that this...
-
CASE STUDY Patient Name Valarie Ramirez Attending Paul F. Buckwalter, MD PATIENT INFORMATION DOB 08/04/1986 Allergies MAN 00-AA-006 Penicillin Other Information Past HX: AB x1 Valarie Ramirez arrives...
-
Petesy Corporation is preparing its Master Budget for 2019. Budget information is as follows: SalesProduction CostOperating Expenses 20191 st Quarter P280,000P192,000P64,000 2 nd Quarter 320,000...
-
A steady flow of 20 m3/s of moist air at TDB = 35iC, TWB = 25iC, 100 kPa (state 1) is dehumidified by first cooling it and condensing out moisture (state 2), then reheating it to 20iC and 50% R.H....
-
Use Householder matrices to convert the following matrices into upper Hessenberg form: (a) (b) (c) 3-2 1025 2410 3201 1134 0101 1213
-
Use translations to graph f. f(x) = x-/2 +1
-
The reaction of chloroethane with water in the gas phase to produce ethanol and hydrogen chloride has Ho = +26.6 kJ mol-1 and So = +4.81 J K-1 mol-1 at 25oC. (a) Which of these terms, if either,...
-
When (S)-2-bromopropanoic acid [(S)-CH3CHBrCO2H] reacts with concentrated sodium hydroxide, the product formed (after acidification) is (R)-2-hydroxypropanoic acid [(R)-CH3CHOHCO2H, commonly known as...
-
Using chair conformational structures (Section 4.11), show the nucleophilic substitution reaction that would take place when trans-1-bromo-4-tert-butylcyclohexane reacts with iodide ion. (Show the...
-
Phantom Consulting Inc. is a small computer consulting business. The company is organized as a corporation and provides consulting services, computer system installations, and custom program...
-
Sam owns a 25% in Spade, LLC. In 2021, Spade reports $100,000 or ordinary income. What is Sams qualified business income (QBI) deduction? answer is 5,000 but please show how to get it
-
crane Inc. common chairs currently sell for $30 each. The firms management believes that it's share should really sell for $54 each. If the firm just paid an annual dividend of two dollars per share...

Study smarter with the SolutionInn App


