Outline a synthesis of (cyclohexylmethyl)methylamine from cyclohexanecarboxylic acid. 010 OH CH-NHCH3 cyclohexanecarboxylic acid (cyclohexylmethyl)methylamine
Question:
Outline a synthesis of (cyclohexylmethyl)methylamine from cyclohexanecarboxylic acid.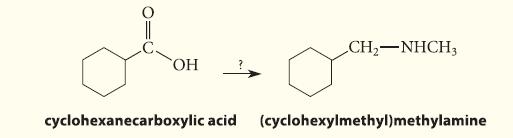
Transcribed Image Text:
010 OH CH₂-NHCH3 cyclohexanecarboxylic acid (cyclohexylmethyl)methylamine
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
Any carboxylic acid derivative used to prepare the amine must contain ...View the full answer

Answered By

JAPHETH KOGEI
Hi there. I'm here to assist you to score the highest marks on your assignments and homework. My areas of specialisation are:
Auditing, Financial Accounting, Macroeconomics, Monetary-economics, Business-administration, Advanced-accounting, Corporate Finance, Professional-accounting-ethics, Corporate governance, Financial-risk-analysis, Financial-budgeting, Corporate-social-responsibility, Statistics, Business management, logic, Critical thinking,
So, I look forward to helping you solve your academic problem.
I enjoy teaching and tutoring university and high school students. During my free time, I also read books on motivation, leadership, comedy, emotional intelligence, critical thinking, nature, human nature, innovation, persuasion, performance, negotiations, goals, power, time management, wealth, debates, sales, and finance. Additionally, I am a panellist on an FM radio program on Sunday mornings where we discuss current affairs.
I travel three times a year either to the USA, Europe and around Africa.
As a university student in the USA, I enjoyed interacting with people from different cultures and ethnic groups. Together with friends, we travelled widely in the USA and in Europe (UK, France, Denmark, Germany, Turkey, etc).
So, I look forward to tutoring you. I believe that it will be exciting to meet them.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Outline a synthesis of each of the following compounds from the indicated starting materials and any other reagents. (a) 1-cyclohexyl-2-methyl-2-prupanol from bromocyclohexane (b) PhNHCH2CH2CH(CH3)2...
-
Outline a synthesis of each of the following compounds from isobutyric acid (2-methylpropanoic acid) and any other necessary reagents. (a) (b) 0 CH) CHC OCH, CCHCH isobutyrophenone
-
Outline a synthesis of phenylethyne from each of the following: (a) (b) (c) (d) Br Br Br
-
Assignment: Based on your reading and analysis of the case study above, address the following items in a detailed essay response of approximately 600 words. Each number below should be addressed...
-
Consider the following incomplete table of merchandisers profit data: Requirement 1. Calculate the missing table values to complete thetable. Cost of Goods Sold Net Sales Gross Profit Sales Sales...
-
Paul Vincelli was disturbed by a telephone call he had just received from an analyst with a major Bay Street investment firm. His company, Madison Avenue Fashions, had just released its third quarter...
-
Are these attitudes of managers described above consistent with another view of dividends discussed earlier?
-
Managers of Tom Brown Distributors are evaluating the compensation system for the company's sales personnel. Currently, the two salespeople have a combined salary of $60,000 per year and earn a 3%...
-
The following information summarizes the standard cost for producing one metal tennis racket frame at Spaulding Industries. In addition, the variances for one month's production are given. Assume...
-
A water-insoluble hydrocarbon A decolorizes a solution of Br 2 in CH 2 Cl 2 . The base peak in the EI mass spectrum of A occurs at m/z = 67. The proton NMR of A is complex, but integration shows that...
-
Isopentenyl pyrophosphate, the starting material for isoprenoid and steroid biosynthesis (Sec. 17.6B), is formed biosynthetically by the decarboxylation reaction of...
-
Subsidiaries Functional Currencies. What would be the functional currency of a self-sustaining foreign subsidiary and an integrated foreign subsidiary?
-
Sketch plane / intersecting plane K. Then draw a line & in plane J that intersects plane Kat a single point. A X C B D E
-
Use a graphing utility to verify any five of the graphs that you drew by hand in Exercises 126. Data from exercise 1-26 1. x + 2y = 8 3. x2y> 10 2. 3x6y 12 4. 2xy > 4
-
The following information pertains to Porter Company for 2011. Ending inventory consisted of 30 units. Porter sold 320 units at \(\$ 30\) each. All purchases and sales were made with cash. Required...
-
In Problems 7780, use a numerical integration routine on a graphing calculator to find the area bounded by the graphs of the indicated equations over the given interval (when stated). Compute answers...
-
Solar Heating, Inc., had the following transactions for 2011: Required a. Determine the quantity and dollar amount of inventory at the end of the year, assuming Solar Heating Inc. uses the FIFO cost...
-
For each of the following Jordan matrices, identify the Jordan blocks. Write down the eigenvalues, the eigenvectors, and the Jordan basis. Clearly identify the Jordan chains. 100 8 (d 0002 011 0130...
-
You are interested in investing and are considering a portfolio comprised of the following two stocks. Their estimated returns under varying market conditions are provided: (note: it is difficult to...
-
The heat of hydrogenation of allene is 298 kJ mol-1, whereas that of propyne is 290 kJ mol-1. (a) Which compound is more stable? (b) Treating allene with a strong base causes it to isomerize to...
-
Although both 1-bromobutane and 4-bromo-1-butene are primary halides, the latter undergoes elimination more rapidly. How can this behavior be explained?
-
Complete the molecular orbital description for the ground state of cyclopentadiene shown at right. Shade the appropriate lobes to indicate phase signs in each molecular orbital according to...
-
A firm purchased a new piece of equipment with an estimated useful life of eight years. The cost of the equipment was $65,000. The salvage value was estimated to be $10,000 at the end of year 8....
-
5. Which of the following is the cheapest for a borrower? a. 6.7% annual money market basis b. 6.7% semi-annual money market basis c. 6.7% annual bond basis d. 6.7% semi-annual bond basis.
-
Waterloo Industries pays 30 percent corporate income taxes, and its after-tax MARR is 24 percent. A project has a before-tax IRR of 26 percent. Should the project be approved? What would your...

Study smarter with the SolutionInn App


