Outline a synthesis of each of the following compounds from the indicated starting material. Begin each synthesis
Question:
Outline a synthesis of each of the following compounds from the indicated starting material. Begin each synthesis with a retrosynthetic analysis.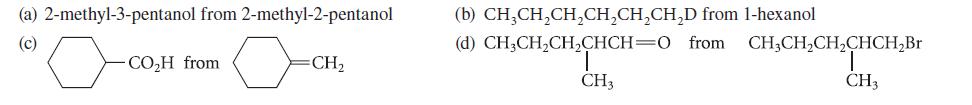
Transcribed Image Text:
(a) 2-methyl-3-pentanol from 2-methyl-2-pentanol (c) -CO₂H from =CH₂ (b) CH3CH₂CH₂CH₂CH₂CH₂D from 1-hexanol (d) CH3CH₂CH₂CHCH=0 from. CH3CH₂CH₂CHCH₂Br CH3 CH3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (6 reviews)
a 2Methyl3pentanol can be prepared by hydroborationoxidation of 2methyl2pentene which ca...View the full answer

Answered By

Lilian Nyambura
Hi, am Lilian Nyambura, With extensive experience in the writing industry, I am the best fit for your writing projects. I am currently pursuing a B.A. in Business Administration. With over 5 years of experience, I can comfortably say I am good in article writing, editing and proofreading, academic writing, resumes and cover letters. I have good command over English grammar, English Basic Skills, English Spelling, English Vocabulary, U.S. English Sentence Structure, U.K. or U.S. English Punctuation and other grammar related topics. Let me help you with all your essays, assignments, projects, dissertations, online exams and other related tasks. Quality is my goal.
4.80+
378+ Reviews
750+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Outline a synthesis for each of the following compounds from the indicated starting material and any other reagents. (a) l-chloro-3, 5-dinitrobenzene from benzene (b) 2-chloro-4,6-dinitrophenol from...
-
Outline a synthesis of each of the following compounds from the indicated starting materials and any other reagents. (a) Benzyl methyl ether from toluene (b) (c) (d) CH2CH O from cyclopentene 7-CO.H...
-
Outline a synthesis of each of the following compounds from the indicated starting materials and any other reagents. (a) 1-cyclohexyl-2-methyl-2-prupanol from bromocyclohexane (b) PhNHCH2CH2CH(CH3)2...
-
The Chief Financial Officer at Ford Motor Company is said to usea hybrid-costing system. Define the hybrid-costing system. Explainthe advantages to this company to use this system. I want a 10 page 2...
-
Refer to the data in PE 6-3. Assume that all of the 80 books sold on credit were sold to a single customer and that the terms of the credit sale were 2/10, n/30. Make the journal entry necessary to...
-
A clay vase on a potter's wheel experiences an angular acceleration of 8.00 rad/s2 due to the application of a 10.0-N m net torque. Find the total moment of inertia of the vase and potter's wheel.
-
Write a first-order model relating E(y) to a. two quantitative independent variables b. four quantitative independent variables c. five quantitative independent variables
-
You want to buy a house within 3 years, and you are currently saving for the down payment. You plan to save $5,000 at the end of the first year, and you anticipate that your annual savings will...
-
Avondale Aeronautics has perpetual preferred stock outstanding with a par value of $100. The stock pays a quarterly dividend of $2.00 and its current price is $123. What is its effective annual rate...
-
The primary alcohol 2-methoxyethanol, CH 3 OCH 2 CH 2 OH, can be oxidized to the corresponding carboxylic acid with aqueous nitric acid (HNO 3 ). The by-product of the oxidation is nitric oxide, NO....
-
The rates of the reactions in Eqs. 10.71ab are increased when the thiol is ionized by a base such as sodium ethoxide. Suggest a mechanism for Eq. 10.71a that is consistent with this observation, and...
-
Use the Chain Rule to find dz/dt or dw/dt. z = sin x cos y, x = t , y = 1/t
-
You have recently taken over daycare center that was under substandard leadership. Currently, the staff is unmotivated, negative, and often absent from work. You notice that there is minimal...
-
Choose an organization from the industry of your choice to discuss, illustrate, and reflect deliberately on the following: Why is it important to distinguish between "group" and "team "? What kinds...
-
The focus of data governance programs, in some capacity, is enterprise-wide data quality standards and processes. If you were a manager focusing on master data: Would you likely meet enterprise-level...
-
1) Identify and explain each component of the ANOVA model. 2) How is the F ratio obtained? 3) What role does the F ratio play?
-
Make a BCG matrix table and place the following products from Apple: iPhone, iPad, iMac, iPod, Apple TV, Apple Watch, AirPod, and HomePod. Briefly describe why you have placed the products in the...
-
For f(x) = x2 + 9 / (x - 3), find each value. (a) f(0.25) (b) f(12.26) (c) f(3)
-
What is EBIT/eps analysis? What information does it provide managers?
-
What stereochemistryantarafacial or suprafacialwould you expect to observe in the following reactions? (a) A photo chemical [1, 5] sigma tropic rearrangement (b) A thermal [4 + 6] cyclo addition (c)...
-
The following thermal isomerization occurs tinder relatively mild conditions. Identify the pericyclic reactions involved, and show how the rearrangementoccurs. CH C- CeH5. C&H5 "CH CD CoH5 CgH5 CEH5...
-
Would you expect the following reaction to proceed in a conrotatory or disrotatory manner? Show the stereochemistry of the Cyclobutene product, and explain youranswer. hv
-
1. Determine the value of the right to use asset and lease liability at commencement of the lease.
-
Problem 22-1 The management of Sunland Instrument Company had concluded, with the concurrence of its independent auditors, that results of operations would be more fairly presented if Sunland changed...
-
Question 4. - Week 9. What are the major competitive issues General Electric faces when managing cooperative strategies? - (7 marks)

Study smarter with the SolutionInn App


