Outline a synthesis of each of the following compounds from either diethyl malonate or ethyl acetate. Because
Question:
Outline a synthesis of each of the following compounds from either diethyl malonate or ethyl acetate. Because the branched amide bases are relatively expensive, you may use them in only one reaction.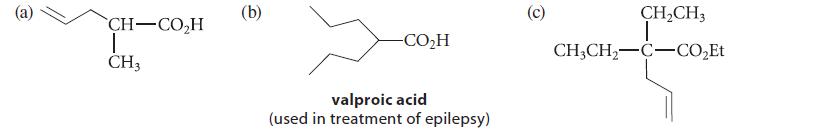
Transcribed Image Text:
CH–COH T CH3 (b) -CO,H valproic acid (used in treatment of epilepsy) (c) CH₂CH3 T CH3CH₂-C-CO₂Et
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
Because part c involves formation of a carboxylic acid with a quaternary acarbon and because ...View the full answer

Answered By

Arshad Ahmad
Well, I am really new to tutoring but I truly believe a good student can be a better teacher. I have always been a topper at school. I passed my Chartered Accountancy at a very young age of 23, a rare feat for most of the students. I am really dedicated to whatever work I do and I am very strict regarding deadlines. i am always committed and dedicated to whatever work allotted to me and I make sure it is completed well within deadline and also I try to give my best in whatever I do. Hope we will have a good time studying together.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Outline a synthesis of each of the following compounds from the indicated strating material and any other reagents. (a) N-(sec-buty1) -N-ethylaniline from chlorobenxene (b) (c) (CH,),C from phenol NH...
-
Outline a synthesis of each of the following compounds from a -keto ester; then show how the -keto ester itself can be prepared. CHj
-
Outline a synthesis of each of the following compounds from the indicated starting materials and any other reagents. (a) 1-cyclohexyl-2-methyl-2-prupanol from bromocyclohexane (b) PhNHCH2CH2CH(CH3)2...
-
Misty Cumbie worked as a waitress at the Vita Caf in Portland, Oregon. The caf was owned and operated by Woody Woo, Inc. Woody Woo paid its servers an hourly wage that was higher than the states...
-
California Pool Supplies inventory data for the year ended December 31, 2012, follow: Assume that the ending inventory was accidentally overstated by $2,400. Requirement 1. What are the correct...
-
Why is it important to protect trademarks from trademark dilution and cyber squatting?
-
Identify the leading economic blocs. LO.1
-
Michael Jacks deposited $500,000 into a bank for 6 months. At the end of that time, he withdrew the money and received $520,000. If the bank paid interest based on continuous compounding: (a) What...
-
Norton Inc. took a physical inventory on 12/31/2019 and determined that $840,000 of goods were on hand. In addition, the following items were not included in the physical count. Norton, Inc....
-
Predict the product formed when the conjugate-base enolate ion of ethyl 2-methylpro panoate (shown in Eq. 22.81) is treated with bromobenzene and a catalytic amount of Pd[P(t-Bu) 3 ] 4 , and explain...
-
Fatty acids are degraded to acetyl-CoA in fatty-acid metabolism. The enzyme that catalyzes this conversion, acyl-CoA acetyl transferase, contains a nucleophilic thiol group in its active site and...
-
George Mayer, CPA, is auditing the allowance for uncollectible accounts. The client has always had collection problems, but management has been uncooperative about booking recommended adjustments to...
-
The financial statements for the Columbia Sportswear Company can be found in Appendix A, and Under Armour, Inc.'s financial statements can be found in Appendix B at the end of this book. Required a....
-
Use the data from SE3-8 to prepare the closing entries for The Decade Company. Close the temporary accounts to income summary. The balance of \(\$ 8,500\) in the retained earnings account is from the...
-
Adjusting Entries The following selected accounts appear in the Birch Company's unadjusted trial balance as of December 31, the end of the fiscal year (all accounts have normal balances): Required...
-
Closing Entries Use the information provided in E3-5A to prepare journal entries to close the accounts using the Income Summary account. After these entries are posted, what is the balance in the...
-
Ceva, Inc. manufactures and services jet engines for air carriers. The engines cost \($10\) to \($40\) million each, depending on the specifications and plane. A 10-year service contract for a single...
-
For each of the following systems (i) (ii) (iii) (a) Find the general real solution. (b) Using the solution formulas obtained in part (a), plot several trajectories of each system. On your graphs,...
-
Saccharin is an artificial sweetener that is used in diet beverages. In order for it to be metabolized by the body, it must pass into cells. Below are shown the two forms of saccharin. Saccharin has...
-
(a) What monobromo allylic substitution products would result from reaction of each of the following compounds with NBS in the presence of peroxides and/or light? (b) In the case of isomeric products...
-
Benzylic radicals, due to the adjacent benzene ring, have even greater possibility for de-localization than allylic radicals. Draw contributing resonance structures that show this delocalization for...
-
When propylbenzene reacts with chlorine in the presence of UV radiation, the major product is 1-chloro-1-phenylpropane. Both 2-chloro-1-phenylpropane and 3-chloro- 1-phenylpropane are minor products....
-
Required information [The following information applies to the questions displayed below.] Dain's Diamond Bit Drilling purchased the following assets this year. Asset Drill bits (5-year) Drill bits...
-
Which of the following partnership items are not included in the self-employment income calculation? Ordinary income. Section 179 expense. Guaranteed payments. Gain on the sale of partnership...
-
Phantom Consulting Inc. is a small computer consulting business. The company is organized as a corporation and provides consulting services, computer system installations, and custom program...

Study smarter with the SolutionInn App


