Predict the product formed when the conjugate-base enolate ion of ethyl 2-methylpro panoate (shown in Eq. 22.81)
Question:
Predict the product formed when the conjugate-base enolate ion of ethyl 2-methylpro panoate (shown in Eq. 22.81) is treated with bromobenzene and a catalytic amount of Pd[P(t-Bu)3]4, and explain the role of the catalyst.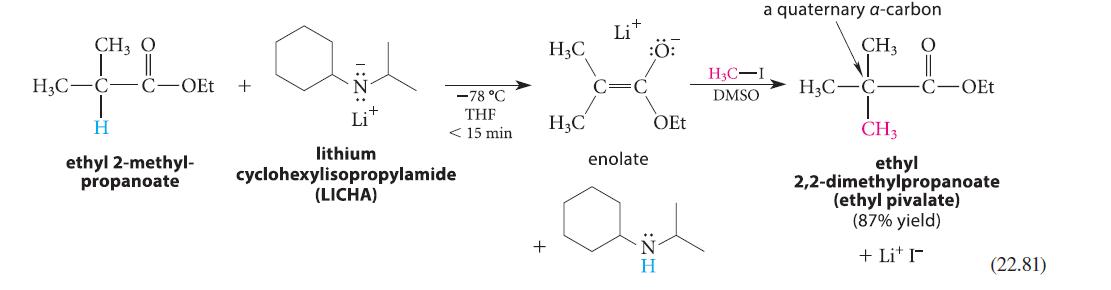
Transcribed Image Text:
CH3 O floo. Agh Lit H3C-C- C -OEt + H ethyl 2-methyl- propanoate -78 °C THF < 15 min lithium cyclohexylisopropylamide (LICHA) H3C H3C + Lit :O: enolate OEt H a quaternary a-carbon CH3 H3C-I DMSO H3C-C- CH3 OEt ethyl 2,2-dimethylpropanoate (ethyl pivalate) (87% yield) + Li+I (22.81)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 42% (7 reviews)
This is a transitionmetal catalyz...View the full answer

Answered By

Emily Grace
With over a decade of experience providing top-notch study assistance to students globally, I am dedicated to ensuring their academic success. My passion is to deliver original, high-quality assignments with fast turnaround times, always striving to exceed their expectations.
4.90+
3+ Reviews
24+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The reactions of ester enolate ions are not restricted to simple alkylations. With this in mind, suggest the structure of the product formed when the enolate ion formed by the reaction of fm-butyl...
-
The compound BD3 is a deuterated form of borane. Predict the product formed when 1-methylcyclohexene reacts with BD3 THF ,followed by basic hydrogen peroxide.
-
A major component of olive oil is glyceryl trioleate. (a) Give the structures of the products expected from the saponification of glyceryl trioleate with excess aqueous NaOH. (b) Give the structure...
-
Francis and Peter are in a partnership sharing profits and losses in the ratio 3:2. The following is their trial balance as at 30 September 2020. particulars D ebit C redit Buildings (cost: RM...
-
Refer back to the California Pool Supplies inventory data in Short Exercise 6-12. Requirement 1. How would the inventory error affect California Pool Supplies cost of goods sold and gross profit for...
-
Could the winner of an Academy Award, or a Heisman Trophy, or any other award with a familiar title, use that trademark as a meta tag for a Web site? Could someone who has not won such an award use...
-
Understand regional integration and economic blocs. LO.1
-
Ted decides to incorporate his medical practice. He uses the cash method of accounting. On the date of incorporation, the practice reports the following balance sheet: All the current liabilities...
-
(Solving for n with nonannual periods ) About how many years would it take for your investment to grow twofold if it were invested at 18 percent compounded monthly? a.If you invest $1 at 18 percent...
-
Indicate whether each of the following compounds could be prepared by a malonic ester synthesis. If so, outline a preparation from diethyl malonate and any other reagents. If not, explain why. (a)...
-
Outline a synthesis of each of the following compounds from either diethyl malonate or ethyl acetate. Because the branched amide bases are relatively expensive, you may use them in only one reaction....
-
Compare and contrast different production methods and accounting systems.
-
From a square whose side has length \(x\), measured in inches, create a new square whose side is 5 in. longer. Find an expression for the difference between the areas of the two squares as a function...
-
Sketch the requested conic sections in Problems 14-23 using the definition. A circle with radius 5
-
Find the present value of the ordinary annuities in Problems 21-32. Amount of Deposit m 23. $250 Frequency n semiannually Rate r 8% Time t 30 yr
-
Characterize the types of investments that are most vulnerable to political risk. Characterize those that are least vulnerable. What factors influence an investments vulnerability? On a scale of 1 to...
-
Refer to the following tree diagram for a two-stage experiment. Find the probabilities in Problems 1-6. \(P(B) \) E E A B C A B C
-
Classify the following systems, and sketch their phase portraits. (a) (b) (c) (d) du dt dv ty lt dt du le dt dv dt dv dt di dv dr LA di
-
Determine the reactions in supports A and D and connections B and C. Sketch its shear and moment diagram and determine the magnitude ankoration of the maximum shear and moment for every member. 18 3...
-
Sketch the 1H NMR spectrum you would expect for the following compound, showing the splitting patterns and relative position of each signal. CI CI Cl CI CI CI
-
List the following radicals in order of decreasing stability:
-
Consider the monochlorination of 2-methylbutane. (a) Assuming that the product mixture was subjected to fractional distillation, which fractions, if any, would show optical activity? (b) Could any of...
-
A stock is expected to pay a dividend of $1.50 at the end of the year (i.e., D 1 = $1.50), and it should continue to grow at a constant rate of 10% a year. If its required return is 14%, what is the...
-
The Hobby Shop has a checking account with a ledger balance of $1,700. The firm has $2,400 in uncollected deposits and $4,200 in outstanding checks. What is the amount of the disbursement float on...
-
An investment will pay you $34,000 in 11 years. If the appropriate discount rate is 6.1 percent compounded daily, what is the present value? (Use 365 days a year. Do not round intermediate...

Study smarter with the SolutionInn App


