Outline a synthesis of hexanal from 1-hexene. CH3CHCHCHCH=CH 1-hexene CHCH,CH,CH,CH,CH=O hexanal
Question:
Outline a synthesis of hexanal from 1-hexene.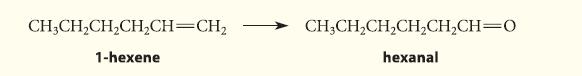
Transcribed Image Text:
CH3CH₂CH₂CH₂CH=CH₂ 1-hexene CHỊCH,CH,CH,CH,CH=O hexanal
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (4 reviews)
To outline a synthesis means to suggest the reagents and conditions required for each step of the sy...View the full answer

Answered By

Subash Murugaih
I am leading expert in this web site couple of years and My clients are much happy with my works and services.
4.60+
309+ Reviews
539+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Write an equation for the synthesis of hexanal from an alcohol.
-
Outline a synthesis of each of the following compounds from the indicated starting materials and any other reagents. (a) Benzyl methyl ether from toluene (b) (c) (d) CH2CH O from cyclopentene 7-CO.H...
-
Outline a synthesis of each of the following compounds from the indicated starting materials and any other reagents. (a) 1-cyclohexyl-2-methyl-2-prupanol from bromocyclohexane (b) PhNHCH2CH2CH(CH3)2...
-
How many vector::push_back() operations are performed between lines 8 and 13 when this code is executed? The size of the input is the number of elements n in the vector v. Replace the question marks...
-
Regina Henry deposited $20,000 in a money market certificate that provides interest of 10% compounded quarterly if the amount is maintained for 3 years. How much will Regina Henry have at the end of...
-
Marvel Media, LLC, has three members: WLKT Partners, Madison Sanders, and Observer Newspaper, LLC. On January 1, 2016, the three members had equity of $200,000, $40,000, and $160,000, respectively....
-
2. What is the profit-sharing ratio for Shin, Miku, and Tora?
-
Part I. Lernout & Hauspie (L& H) was the worlds leading provider of speech and language technology products, solutions, and services to businesses and individuals worldwide. Both Microsoft and Intel...
-
a . Global used $ 2 0 . 0 million of its available cash to repay $ 2 0 . 0 million of its long - term debt. b . A warehouse fire destroyed $ 5 . 0 million worth of uninsured inventory. c . Global...
-
Verify that the acid-catalyzed hydration of 2-methylpropene is neither an oxidation nor a reduction.
-
What alkene(s) are formed in the acid-catalyzed dehydration of each of the following alcohols? (a) 3-methyl-3-heptanol (b) OH PhCHCHPh
-
Would the regression in Equation (4.9) in chapter 4 be useful for predicting test scores in a school district in Massachusetts? Why or why not? Equation (4.9) TestScore 698.9 2.28 STR,
-
We are writing a business plan about expanding Robinson Development Group's business into Mexico. They do residential as well as business development in Virginia and have branched out on the East...
-
Political ideologies can influence trade. Although Russia's constitution lists it as a Federal Democratic State, many news outlets call it a "Fake Democracy". Now that Russia has invaded Ukraine, the...
-
A facultative oxidation pond is to be designed for a community of 5000 people. Summer wastewater flow is 2000 m 3 /d, and the BOD 5 is 180 g/m 3 . Winter flow and BOD 5 values are 6000 m 3 /d and 90...
-
Watch the video "Black Diamond: Managing in a Global Environment" https://youtu.be/lc29Ro9TOKg Describe at least two environmental factors that affect this business and summarize how the managers are...
-
In social media, one size does not fit all. Social media includes traditional platforms such as Facebook and Instagram, but it also includes podcasts, blogs, and video mediums. Each platform and...
-
Find the convergence set for We know that (xn / n! converges for all x. Why can we conclude that limn( xn / n! = 0 for all x? r" 2
-
Danielle has an insurance policy with a premium of $75 per month. In September she is in an accident and receives a bill worth $2990 for the repair of her own property. Her deductible is $250 and her...
-
Predict the product(s) of the followingreactions: (b) 1. Nat "OEt Co (a) 2. CH3I eat (c) Br2, PBr3 H20 CH-CH2C (d) " NaOH, H20 12
-
Which, if any, of the following compounds can be prepared by a malonic ester synthesis? Show the alkyl halide you would use in each case. (a) Ethyl pentanoate (b) Ethyl 3-rnethylbutanoate (c) Ethyl...
-
Which, if any, of the following compounds can be prepared by an acetoacetic ester synthesis?Explain. (a) Br. (c) (b) CH CH - CH
-
Suppose First Fidelity Bank engaged in the following transactions: (Click the icon to view the transactions.) Journalize the 2018 and 2019 transactions on First Fidelity's books. Explanations are not...
-
Financial data for Joel de Paris, Inc., for last year follow: Joel de Paris, Inc. Balance Sheet Beginning Balance Ending Balance Assets Cash Accounts receivable Inventory Plant and equipment, net...
-
Supply costs at Coulthard Corporation's chain of gyms are listed below: March April May June July August September October November Client-Visits 11,666 11,462 11,994 13,900 11,726 11, 212 12,006...

Study smarter with the SolutionInn App


