What alkene(s) are formed in the acid-catalyzed dehydration of each of the following alcohols? (a) 3-methyl-3-heptanol (b)
Question:
What alkene(s) are formed in the acid-catalyzed dehydration of each of the following alcohols?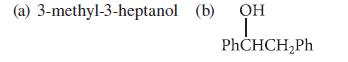
Transcribed Image Text:
(a) 3-methyl-3-heptanol (b) OH ī PhCHCH₂Ph
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
a b CH3 CH3 CH I CHCHCCHCHCHCH CHCHCCHCHCHCH CHCHCCHCHCHCH C ...View the full answer

Answered By

Michael Mulupi
I am honest,hardworking, and determined writer
4.70+
72+ Reviews
157+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
What three alkenes are formed in the acid-catalyzed dehydration of 2-pentanol?
-
What three alkenes are formed in the acid-catalyzed dehydration of 2-pentanol?
-
Identify the major alkene product(s) in part (a) of Problem 10.4. Problem 10.4. What alkene(s) are formed in the acid-catalyzed dehydration of each of the following alcohols?
-
Using monthly data for Meta since its IPO date of 31/12/2019, estimate equity beta for Meta. Compare your estimate of Metas equity beta with Facebooks beta provided by Yahoo Finance and comment on...
-
How should correction of errors be reported in the financial statements?
-
Apex Inc. reports the following for a recent year: Income from continuing operations before income tax ............$1,000,000 Extraordinary property loss from hurricane ............................
-
E 16-11 Recording partner retirementRevaluation case Capital balances and profit-sharing percentages for the Achmad, Fakhry, and Fatimah partnership on December 31, 2016, just before the retirement...
-
The Hershey Company is one of the worlds leading producers of chocolates, candies, and confections. It sells chocolates and candies, mints and gums, baking ingredients, toppings, and beverages....
-
Zeta Gaming company has an opportunity to purchase a video game phone app that will cost $150,000. Zeta expects the demand for the app to start strong but to diminish as people tire of the game. The...
-
Outline a synthesis of hexanal from 1-hexene. CH3CHCHCHCH=CH 1-hexene CHCH,CH,CH,CH,CH=O hexanal
-
Use oxidation numbers to verify that the transformation in Eq. 10.39 (also shown below) is an oxidation. HC-CH-OH ethanol O || HC-C-OH acetic acid (10.39)
-
There are two errors in the following trial balance: (1) One account has been placed in the wrong column, and (2) There is a transposition error in the balance of the L. Bourque, Capital account....
-
Propose how these mechanisms can be used to build a strategic business partnership, close the gap between management / leadership and employees while building a cohesive culture that adds value,...
-
1.What risks does the company face? 2. What is role for ERM at Swissgrid or most any company? 3. What risk management processes has Meyer installed at Swissgrid? Assess their strengths and...
-
Elizabeth's Country Wares How many workers does Elizabeth have and what does each of them do? What type of work does Elizabeth do for the CP product line? How long does it take to do the underglazing...
-
Do you support the policy of not allowing some Chinese nationals to attend graduate school in the United States because of national security concerns?
-
Using your product or service name or category, do a search using the following phrase: Find a (insert the name of your product or service here...) near me. For instance, using my Mobile Notary...
-
In Problems 1-4, find the convergence of set for the given power series? 1. 2. 3. 4. (n-1)!
-
31. What is the income that can be received over 15 years from $500,000 earning 6% annually? 32. What is the semiannual payment required to retire $50,000 in debt over 5 years at 8% compounded...
-
How would you prepare the following ketones using an acetoacetic estersynthesis? (a) (b) CH-H CH-CH CH2CH2CHCH3 CH3 CH
-
How would you prepare the following compounds using either an acetoacetic ester synthesis or a malonic estersynthesis? (d) (c) ( (a) H CHO2Et H H2H,H3 "CH Et
-
Which of the following substances would undergo the halo form reaction? (a) CH3COCH3 (b) Acetophenone (c) CH3CH2CHO (d) CH3CO2H (e) CH3C N
-
Chapter o Homew ebook 50,000-unit production quantity: $ 227,049 7 70,000-unit production quantity: $ 66,751 d. In addition to mean profit, what other factors should FTC consider in determining a...
-
Diamond makes downhill ski equipment. Assume that comic has offered to produce ski poles for Diamond for $20 per pair Diamond needs 200,000 pairs of poles per period Diamond can only avoid 5150,000...
-
17? Which of the following statement is true Select one: a. All evidence must have the same level of reliability b. All evidence must have the same level of persuasiveness C. All are false d....

Study smarter with the SolutionInn App


