How would you prepare the following compounds using either an acetoacetic ester synthesis or a malonic estersynthesis?
Question:
How would you prepare the following compounds using either an acetoacetic ester synthesis or a malonic estersynthesis?
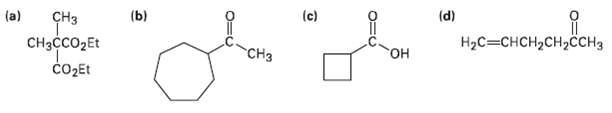
Transcribed Image Text:
(d) (c) (ы (a) ҫHз CHзссO2Et Hас —снсH2сH,ссH3 "CHз он соДEt
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 56% (16 reviews)
Use a malonic ester synthesis if the product you want is an asubstituted carboxylic acid or derivati...View the full answer

Answered By

HILLARY KIYAYI
I am a multi-skilled, reliable & talented Market analysis & Research Writer with a proven ability to produce Scholarly Papers, Reports, Research and Article Writing and much more. My ultimate quality is my English writing/verbal skill. That skill has proven to be the most valuable asset for project writing, Academic & Research writing, Proofreading, HR Management Writing, business, sales, and a variety of other opportunities.
4.80+
24+ Reviews
60+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
How could you prepare the following compounds using a starting material containing no more than three carbon atoms? a. b. CH3 CH CH CHCHCH O Br CH CH2CH2CCH3
-
How would you prepare the following compounds fromCyclohexanone? la) (b) o C6H5CH, CHC&H5 CH2CH2CN (c) (d) CH2CH=CH2 CO2Et
-
How would you prepare the following compounds from 1-phenylethanol? (a) Methyl 1-phenyl ethyl ether (b) Phenylepoxyethane (c) tert-Butyl 1-phenylethyl ether (d) 1-Phenylethanethiol
-
The following summarized statement of profit or loss has been extracted from the financial statements of Gwembe Mining Corporation, a Zambian resident company which engaged in open cast mining...
-
Following is a letter to a customer who demanded a brand-new replacement Droid smart phone under her wireless phone protection plan. Analyze the message. List its weaknesses, and then outline an...
-
What is the government expenditure equation? Explain the three budget positions.
-
*Using Equation 9.12 (page 214), show that the maximized likelihood for the linear model can be written as L 2e e0 e n & '(n=2
-
On November 30, Windsor Party Planners had a $43,000 balance in Accounts Receivable and a $3,700 credit balance in Allowance for Uncollectible Accounts. During December, Windsor Party Planners made...
-
apter 11 homework Saved Help Save & Exit Check my 7 30 Required information [The following information applies to the questions displayed below.] On January 1, 2021, the Allegheny Corporation...
-
On December 31, Year 1, the West Corporation estimated that $6,000 of its receivables might not be collected. At the end of Year 1, the unadjusted balances of Accounts Receivable and Allowance for...
-
How would you prepare the following ketones using an acetoacetic estersynthesis? (a) (b) CH-H CH-CH CH2CH2CHCH3 CH3 CH
-
Which of the following substances would undergo the halo form reaction? (a) CH3COCH3 (b) Acetophenone (c) CH3CH2CHO (d) CH3CO2H (e) CH3C N
-
LO3 Determine the adjusted basis of each of the following assets: a. Andre purchased a parcel of land three years ago for $17,000. In the current year, the adjoining property owner sues him, claiming...
-
Draw a bar graph for each data set in Problems 32-35. Data set \(\mathrm{D}\) Data set A: The annual wages of employees at a small accounting firm are given in thousands of dollars. 35 25 25 16 14 1...
-
Draw a line graph for each data set in Problems 36-39. Data set A Data set A: The annual wages of employees at a small accounting firm are given in thousands of dollars. 25 16 25 25 14 18 1 2 2 2...
-
For each of the angles shown: (i) Estimate its size (ii) Measure it and check how good your estimate was. Aim for your estimate to be within 10 of the actual angle. a. b. c. d. e. f.
-
For the quasispin model of Problem 31.1 , find the eigenvalues of $s_{0}^{(m)}$ for the levels labeled by $m$. Show that the system has a total quasispin $S$ that is the vector sum of quasispins for...
-
A sole proprietorship was started on January 1, 2005, when it received \($60,000\) cash from Mark Pruitt, the owner. During 2005, the company earned \($40,000\) in cash revenues and paid \($19,300\)...
-
What are the critical issues that a company must consider when trying to match its staffing to its strategy? AppendixLO1
-
What is the amount of total interest dollars earned on a $5,000 deposit earning 6% for 20 years?
-
A molecule contains three identical polar bonds in a trigonal planar molecular geometry. Is the molecule polar? (a) Yes (b) No (c) Unable to determine whether the molecule is polar without more...
-
When a suspension of 2, 4, 6-tribromophenol is treated with an excess of bromine water, the white precipitate of 2, 4, 6-tribromophenol disappears and is replaced by a precipitate of a yellow...
-
Outline a synthesis for each of the following compounds from the indicated starting material and any other reagents. (a) l-chloro-3, 5-dinitrobenzene from benzene (b) 2-chloro-4,6-dinitrophenol from...
-
Complete each reaction given in Fig. P18.65, pp. 882-883, by giving the major organic product(s), and explain your reasoning. "No reaction" may be an appropriate response. (a) (b) (c) (d) (e) (f)...
-
question 6 Timely Inc. produces luxury bags. The budgeted sales and production for the next three months are as follows july. august september Sales, in units 1,115. 1229. 1302 Production. in units...
-
On May 12 Zimmer Corporation placed in service equipment (seven-year property) with a basis of $220,000. This was Zimmer's only asset acquired during the year. Calculate the maximum depreciation...
-
Power Manufacturing has equipment that it purchased 7 years ago for $2,550,000. The equipment was used for a project that was intended to last for 9 years and was being depreciated over the life of...

Study smarter with the SolutionInn App


