How would you prepare the following ketones using an acetoacetic estersynthesis? (a) (b) CH-H CH-CH CH2CH2CHCH3 CH3
Question:
How would you prepare the following ketones using an acetoacetic estersynthesis?
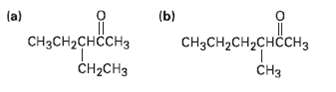
Transcribed Image Text:
(a) (b) CнзсH-снссHз CH-CHз CH2CH2CHCH3 CH3 CHз
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 70% (17 reviews)
12 Na OEt CHCH3 22 CHCHBr CO Et CH3CH2CCCH3 COEt 2 Na...View the full answer

Answered By

Abigael martinez
I have been a tutor for over 3 years and have had the opportunity to work with students of all ages and backgrounds. I have a strong belief that all students have the ability to learn and succeed if given the right tools and support. I am patient and adaptable, and I take the time to get to know each student's individual learning style in order to best support their needs. I am confident in my ability to help students improve their grades and reach their academic goals.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
How could you prepare the following ketones by reaction of an acid chloride with a lithium diorganocopper reagent? (b) (a)
-
Using any alkyne needed, how would you prepare the following alkenes? (a) Trans-2-Octenc (b) Cis-3-Heptcne (c) 3-Methyl-1-pentene
-
How would you prepare the following diols? (b) (a)
-
Relationship of the Balanced Scorecard to activity-based costing Explain how an activity-based costing model can be linked to a Balanced Scorecard approach.
-
What distinguishes rational, emotional, and dual appeals in persuasion?
-
What were three important regulatory powers that the Fed gained from the passage of the Financial Regulatory Reform Act of 2010? Explain each briefly.
-
Using Duncans regression of occupational prestige on income and education, and performing the necessary calculations, verify that the omnibus null hypothesis H0: 1 2 0 can be tested as a general...
-
National Orthopedics Co. issued 9% bonds, dated January 1, with a face amount of $500,000 on January 1, 2011. The bonds mature in 2014 (4 years). For bonds of similar risk and maturity the market...
-
A 3% convertible corporate bond with a face value of $1,000 and 20 years remaining to maturity trades to yield 0.5%. Its conversion ratio is 40. The market value of the bond is equal to 104% of the...
-
Joseph Randal has just been named the director of alumni relationships at a local university. Alumni donations are an important source of revenue for colleges and universities. If administrators...
-
Which, if any, of the following compounds can be prepared by an acetoacetic ester synthesis?Explain. (a) Br. (c) (b) CH CH - CH
-
How would you prepare the following compounds using either an acetoacetic ester synthesis or a malonic estersynthesis? (d) (c) ( (a) H CHO2Et H H2H,H3 "CH Et
-
The Salem Company bond currently sells for $867.59, has a 6% coupon rate and a $1,000 par value, pays interest annually, and has 15 years to maturity. a. Calculate the yield to maturity (YTM) on this...
-
Arrow Company processes a food seasoning powder through its Compounding and Packaging departments. In the Compounding Department, direct materials are added at the beginning of the process, and...
-
The 2017 financial statements of LVMH Moet Hennessey Louis Vuitton S.A. are presented in Appendix C at the end of this book. LVMH is a Paris-based holding company and one of the world's largest and...
-
Repeat Problem 10.E1, except design a packed column using 1-in. metal Pall rings. Do the calculations at the top of the column. Approximate HETP for ethanol-water is \(0.366 \mathrm{~m}\). At...
-
We are separating an ethanol-water mixture in a column operating at atmospheric pressure with a total condenser and a partial reboiler. Constant molal overflow (CMO) can be assumed, and reflux is a...
-
Corporate Social Responsibility Problem The Global Reporting Initiative (GRI) is a networkbased organization that has pioneered the development of the world's most widely used sustainability...
-
Utilize an action planning framework to implement an organizations MBO and TQM initiatives. AppendixLO1
-
How many years will it take a $700 balance to grow into $900 in an account earning 5%?
-
According to valence bond theory, which kind of orbitals overlap to form the PCl bonds in PCl 5 ? a) P(sp) -Cl(p) b) P(spd) - Cl(s) c) P(sp) -Cl(s) d) P(spd) - Cl(p)
-
Vanillin is the active component of natural vanilla flavoring. OCH vanillin
-
1-Haloalkynes are known compounds. (a) Would 1-bromo-2-phenylacetylene (Br-C==CPh) be likely to undergo an SN2 reaction? Explain. (b) Would the same compound be likely to undergo an SN1 reaction?...
-
Ferulic acid is a potent antioxidant found in tomatoes and other vegetables that protects against oxidative stress in neuronal cells, and is thus of some interest in research on Alzheimer's disease....
-
Choose two stocks from the same industry to minimize the influence of other confounding factors. You choose the industry that you are relatively more familiar with, and then estimate the implied...
-
why should Undertake research to review reasons for previous profit or loss?
-
A pension fund's liabilities has a PV01 of $200 million. The plan has $100 billion of assets with a weighted average modified duration of 8. The highest duration bond that the plan can invest in has...

Study smarter with the SolutionInn App


