How would you prepare the following compounds fromCyclohexanone? la) (b) o C6H5CH, CHC&H5 CH2CH2CN (c) (d) CH2CH=CH2
Question:
How would you prepare the following compounds fromCyclohexanone?
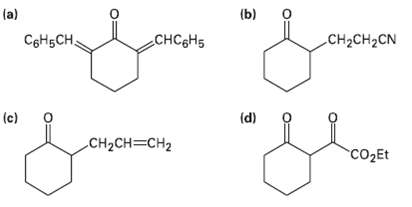
Transcribed Image Text:
la) (b) o C6H5CH, CHC&H5 CH2CH2CN (c) (d) CH2CH=CH2 CO2Et
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 90% (10 reviews)
So 1 NaOH CsH5CH EtOH 2 CoHsCHO ...View the full answer

Answered By

Fahmin Arakkal
Tutoring and Contributing expert question and answers to teachers and students.
Primarily oversees the Heat and Mass Transfer contents presented on websites and blogs.
Responsible for Creating, Editing, Updating all contents related Chemical Engineering in
latex language
4.40+
8+ Reviews
22+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
How would you prepare the following compounds starting with an appropriate carboxylic acid and any other reagents needed? (Reddish brown =Br.) (a) (b)
-
How would you prepare the following compounds using either an acetoacetic ester synthesis or a malonic estersynthesis? (d) (c) ( (a) H CHO2Et H H2H,H3 "CH Et
-
How would you prepare the following compounds from toluene? A diazonio replacement reaction is needed in someinstances. (a) (b) (c) NH2 CH2NH2 H
-
The emergency room of the community hospital in Farmburg has one receptionist, one doctor, and one nurse. The emergency room opens at time zero, and patients begin to arrive some time later. Patients...
-
Coke and Pepsi have sustained their market dominance for nearly a century. General Motors and Ford have recently been hard hit by competition. What is different about the product/market situation in...
-
A 2.0 kg object is moving to the right with a speed of 1.0 m/s when it experiences the force shown in FIGURE EX11.8. What are the objects speed and direction after the force ends? F, (N) 1.0 s - t...
-
How much of a carried interest will the deal support?
-
Two items are omitted in each of the following four lists of income statement data. Determine the amounts of the missing items, identifying them byletter. igl Sas0,000 Sales Seles returns and...
-
Which one of the following performance measures is the Sharpe ratio? Select one: A. average excess return to beta ratio B. alpha to standard deviation of residuals ratio C. average return minus...
-
John is a regional manager of a chain of stores that sell computer equipment and accessories, mainly based out of town in retail parks.A new manager has been appointed to one of the stores, which...
-
Fill in the missing reagents a?h in the following scheme: CO2Et CH3 CO2Et 2. f 1. c 2. d 1. a 2. b 1. g 1. e 2. h CO2Et
-
Leucine, one of the twenty amino acids found in proteins, is metabolized by a pathway that includes the following step. Propose amechanism. "02C "C H3C SCOA SCOA 3-Hydroxy-3-methyl- glutaryl CoA...
-
A classified income statement consists of only two categories of items, revenues and expenses. 56k
-
inverse function of f ( x ) = 9 - 8 e ^ x
-
Let = <3,2,-1) = < 1,3 -> W=
-
1. This is a group assignment, and the lecturer will create and finalize assignment groups in week 3/4. (4-5 members in each group). 2. Identify a problem (only one problem relating to OB) in an...
-
Fromthefollowinginformation, preparejournalentriestodistributetransportationexpenses(ontheaverage rate permilepermonthmethod)andstoresexpenses. Truckmileageduringthemonth:...
-
2 Staffing at the Optimal Utilization A large theme park is attempting to staff its check-in desks. Currently, the arrival rate is A = 364.5 customers per hour, and each server can check-in p=81...
-
Managers cannot motivate employees; only employees can motivate themselves. A. True B. False
-
How do individual companies respond to economic forces throughout the globe? One way to explore this is to see how well rates of return for stock of individual companies can be explained by stock...
-
Draw the Lewis structure for CO with an arrow representing the dipole moment. Refer to Figure 10.10 to estimate the percent ionic character of the CO bond. Percent ionic character 100 75 50 25 0. HI...
-
Give the systematic (IUPAC) names of the following alkenes. (a) (b) (c) (d) (e) (f) (g) (h) (i) (j) CH2 CHCH2CH(CH3)2 CH3(CH2)3-C-CH2CH3 CH2 CH CHCH2 CH CH2 CH2=C=CH-CH-CH2 " CH3 CH3
-
A chemist allows some pure (2S,3R)-3-bromo-2,3-diphenylpentane to react with a solution of sodium ethoxide (NaOCH2CH3) in ethanol. The products are two alkenes: A (cis-trans mixture) and B, a single...
-
The energy difference between cis- and trans-but-2-ene is about 4 kJ mol; however, the trans isomer of 4,4-dimethylpent-2-ene is nearly 16 kJ mol more stable than the cis isomer. Explain this large...
-
As a Financial Analyst in the Finance Department of Zeta Auto Corporation they are seeking to expand production. The CFO asks you to help decide whether the firm should set up a new plant to...
-
Chapter 4 When an Auditor finds misstatements in entities financial statements which may be the result of fraudulent act, what should be the role of an auditor under that situation? (2 Points)
-
Suppose the following input prices are provided for each year: Required: $

Study smarter with the SolutionInn App


