Predict the major product in each case that would be obtained when the following epoxide reacts with
Question:
Predict the major product in each case that would be obtained when the following epoxide reacts with water under
(a) Basic conditions;
(b) Acidic conditions.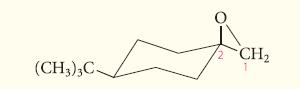
Transcribed Image Text:
(CH3)3C- CH₂
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
As the preceding summary suggests when attempting to predict the products of an epoxide ringopening ...View the full answer

Answered By

Shem Ongek
I am a professional who has the highest levels of self-motivation. Additionally, I am always angled at ensuring that my clients get the best of the quality work possible within the deadline. Additionally, I write high quality business papers, generate quality feedback with more focus being on the accounting analysis. I additionally have helped various students here in the past with their research papers which made them move from the C grade to an A-grade. You can trust me 100% with your work and for sure I will handle your papers as if it were my assignment. That is the kind of professionalism that I swore to operate within. I think when rating the quality of my work, 98% of the students I work for always come back with more work which therefore makes me to be just the right person to handle your paper.
4.80+
174+ Reviews
426+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Predict the major product (or products) that would be obtained when each of the following compounds is nitrated: (a) (b) (c) OH CF CN SO3H OCH3 NO2
-
Predict the major product (or products) formed when each of the following reacts with a mixture of concentrated HNO3 and H2SO4. (a) (b) (c) 4-Chlorobenzoic acid (d) 3-Chlorobenzoic acid (e)...
-
The S N 2 reaction between a Grignard reagent and an epoxide works reasonably well when the epoxide is ethylene oxide. However, when the epoxide is substituted with groups that provide steric...
-
A manufacturing company reports the following information for the month of May. Note: Assume all raw materials were used as direct materials. Activities for May Advertising expense Raw materials...
-
Domitian Corporation accounts for uncollectible accounts receivable using the allowance method. As of December 31, 2011, the credit balance in Allowance for Bad Debts was $170,000. During 2012,...
-
On a distant planet, golf is just as popular as it is on earth. A golfer tees off and drives the ball 3.5 times as far as he would have on earth, given the same initial velocities on both planets....
-
4 Select one strength, one weakness, one opportunity, and one threat from the Ben & Jerrys SWOT analysis shown in Figure 27. Suggest an action that a B&J marketing manager might take to address each...
-
Dorina Company makes cases of canned dog food in batches of 1,000 cases and sells each case for $15. The plant capacity is 50,000 cases; the company currently makes 40,000 cases. DoggieMart has...
-
Requirement 2. How much net income or net loss did Sign Company earn for the year ended 31? How can you tell? Start by identifying the amount of net income or loss for the year ended 31. (Enter a...
-
What is the stereochemistry of the 2,3-butanediol formed when meso-2,3-dimethyloxirane reacts with aqueous sodium hydroxide?
-
Arrange the ions in the following list in order of increasing acidity, and explain your reasoning. | A H3C B CH3 H3C C H D
-
The reconciling item in a bank reconciliation that will result in an adjusting entry by the depositor is: (a) outstanding checks. (b) deposit in transit. (c) a bank error. (d) bank service charges.
-
What are factors which hamper the promotion of an entrepreneurial culture in South Africa?
-
Please Help P/R End Date 2/8/2019 Company Name: Prevosti Farms and Sugarhouse Check Date 2/13/2019 Tax Name M/S # of W/H Hourly Rate or Period # of Regular # of Overtime # of Holiday Wage Hours Hours...
-
Read the description of following adjustments that are required at the end of the accounting period for Hubbard Repair Services, a new firm. Determine the account and amount to be debited and the...
-
Jamie Lee and Ross, now 57 and still very active, have plenty of time on their hands now that the triplets are away at college. They both realized that time has just flown by; over twenty-four years...
-
Here are summary statistics for randomly selected weights of newborn girls: n = 36, x = 3180.6 g, s = 700.5 g. Use a confidence level of 99% to complete parts (a) through (d) below. a. Identify the...
-
Verify the following are identities. (a) sin u/csc u + cos u / sec u = 1 (b) 1 - cos2 x) (1 + cot2 x) = 1 (c) sin t (csc t - sin t) = cos2 t (d) 1 - csc2 t/ csc2 t = - 1 / sec2 t
-
Under what conditions is the following SQL statement valid?
-
Draw the complete structure of the deoxyribonuclcotide sequence from which the mRNA codon in Problem 28.24 was transcribed. Problem 28.24 UAC is a codon for tyrosine it was transcribed from ATG of...
-
Give an mRNA sequence that will code for synthesis of metenkephalin. Tyr-Gly-Gly- Phe-Met
-
Give an mRNA sequence that will code (or the synthesis of angiotensin II. Asp-Arg-Val-Tyr-IIe-His-Pro-Phe
-
ABC Corporation has an activity - based costing system with three activity cost pools - Machining, Setting Up , and Other. The company's overhead costs, which consist of equipment depreciation and...
-
Consolidated Balance Sheets - USD ( $ ) $ in Thousands Dec. 3 1 , 2 0 2 3 Dec. 3 1 , 2 0 2 2 Current assets: Cash and cash equivalents $ 9 8 , 5 0 0 $ 6 3 , 7 6 9 Restricted cash 2 , 5 3 2 Short -...
-
How does corporate governance contribute to investor confidence and stakeholder trust? Accounting

Study smarter with the SolutionInn App


