Rank the following compounds in order of increasing acidity. Explain your answers. OH A OCH3 OH +
Question:
Rank the following compounds in order of increasing acidity. Explain your answers.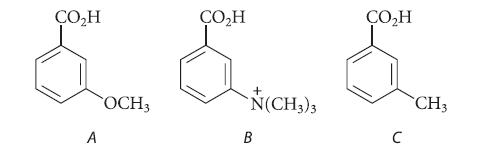
Transcribed Image Text:
ÇO₂H A OCH3 ÇO₂H + N(CH3)3 B CO₂H C CH3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
The trimethylammonium substituent in B has the greatest acidstrengthening polar ...View the full answer

Answered By

PALASH JHANWAR
I am a Chartered Accountant with AIR 45 in CA - IPCC. I am a Merit Holder ( B.Com ). The following is my educational details.
PLEASE ACCESS MY RESUME FROM THE FOLLOWING LINK: https://drive.google.com/file/d/1hYR1uch-ff6MRC_cDB07K6VqY9kQ3SFL/view?usp=sharing
3.80+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Rank the following compounds in order of increasing reactivity (least reactive first) in an SN1 solvolysis reaction in aqueous acetone. Explain your answers. (The structure of tert-cumyl chloride is...
-
Rank the following compounds in order of increasing reactivity in bromination. In each case, indicate whether the principal monobromination products will be the ortho and para isomers or the meta...
-
Shock Electronics sells portable heaters for $35 per unit, and the variable cost to produce them is $22. Mr. Amps estimates that the fixed costs are $97,500. a. Compute the break-even point in units....
-
Suppose a Bubba store purchases $61,000 of womens sportswear on account from Tomas on July 1, 2012. Credit terms are 2/10, net 45. Bubba pays electronically, and Tomas receives the money on July 10,...
-
What is the entry for a loan to an employee?
-
Libra plc has an estimated terminal value (representing cash flows beyond the planning horizon) of 100 million. What is the present value of this figure assuming a discount rate of 12 per cent and a...
-
1. A principal source of revenue for hospitals is from patient services. Patient services revenue for hospitals is recorded at: a. Amounts actually billed to patients b. The hospitals full...
-
Calculate the target inventory for a service level of 90% _______________________________________________ Q4. (25 points) The 40 week demand data for a product is given below. Replenishment cycle is...
-
Explain why all efforts to synthesize a carboxylic acid containing the isotope oxygen-18 at only the carbonyl oxygen fail and yield instead a carboxylic acid in which the labeled oxygen is...
-
Draw the structures and give the names of all the dicarboxylic acids with the formula C 6 H 10 O 4 . Indicate which are chiral, which would readily form cyclic anhydrides on heating, and which would...
-
Albert Einstein said that compound interest was ". . . the most powerful thing I have ever witnessed." Work through the following exercises to discover a pattern Einstein discovered which is now...
-
Consider the project information in the table below: Draw and analyze a project network diagram to answer the following questions: a. If you were to start on this project, which are the activities...
-
Lacey, Inc., had the following sales and purchase transactions during 2011. Beginning inventory consisted of 80 items at \(\$ 120\) each. Lacey uses the FIFO cost flow assumption and keeps perpetual...
-
Refer to the Camp Sunshine data presented in E5-9. Required: 1. Perform a least-squares regression analysis on Camp Sunshines data. 2. Using the regression output, create a cost equation (Y = A + BX)...
-
The following information pertains to the first year of operation for Sonic Boom Radios, Inc. Required: Prepare Sonic Booms full absorption costing income statement and variable costing income...
-
Jane Crawford, the president of Crawford Enterprises, is considering two investment opportunities. Because of limited resources, she will be able to invest in only one of them. Project A is to...
-
True or false: If A has Jordan canonical form J, then A2 has Jordan canonical J2.
-
Refer to the situation described inBE 18-13, but assume a 2-for-1 stock split instead of the 5% stock dividend. Prepare the journal entry to record the stock split if it is to be effected in the form...
-
Although Hückel's rule (Section 14.7) strictly applies only to monocyclic compounds, it does appear to have application to certain bicyclic compounds, if one assumes use of resonance...
-
(a) In 1960 T. Katz (Columbia University) showed that cyclooctatetraene adds two electrons when treated with potassium metal and forms a stable, planar dianion, C8H82- (as the dipotassium salt): Use...
-
Although none of the [10]annulenes given in Section 14.7B is aromatic, the following 10 p-electron system is aromatic: What factor makes this possible?
-
Hite corporation intends to issue $160,000 of 5% convertible bonds with a conversion price of $40 per share. The company has 40,000 shares of common stock outstanding and expects to earn $600,000...
-
Your portfolio has a beta of 1.17, a standard deviation of 14.3 percent, and an expected return of 12.5 percent. The market return is 11.3 percent and the risk-free rate is 3.1 percent. What is the...
-
Slow Roll Drum Co. is evaluating the extension of credit to a new group of customers. Although these customers will provide $198,000 in additional credit sales, 13 percent are likely to be...

Study smarter with the SolutionInn App


