Tert-butyl esters can be prepared by the acid-catalyzed reaction of methylpropene (isobutylene) with carboxylic acids. Suggest a
Question:
Tert-butyl esters can be prepared by the acid-catalyzed reaction of methylpropene (isobutylene) with carboxylic acids.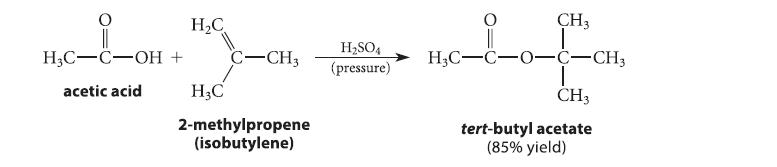
Suggest a mechanism for this reaction that accounts for the role of the acid catalyst.
Transcribed Image Text:
H3C-C-OH acetic acid H₂C + C-CH3 H3C 2-methylpropene (isobutylene) H₂SO4 (pressure) CH3 Lof H₂C-C-0-C-CH3 CH3 tert-butyl acetate (85% yield)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 40% (5 reviews)
In this reaction the carboxylic acid is alkylated b...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The catalyst [Rh(Ph 2 PCH 2 CH 2 PPh 2 )] + can be prepared by the reaction of [Rh(nbd)(Ph 2 PCH 2 CH 2 PPh 2 )] + (nbd = 25.37) with two equivalents of H 2 . In coordinating solvents [Rh(Ph 2 PCH 2...
-
The hydrolysis of the ester shown here is catalyzed by morpholine, a secondary amine. Propose a mechanism for this reaction. (The pKa of the conjugate acid of morpholine is 9.3, so morpholine is too...
-
(a) Organolithium reagents such as methyllithium (CH 3 Li) react with carboxylic acids to give ketones. Two equivalents of the lithium reagent are required, and the ketone does not react further....
-
Thomas Gilbert and Susan Bradley formed a professional corporation called Financial Services Inc.A Professional Corporation, each taking 50 percent of the authorized common stock. Gilbert is a CPA...
-
The unadjusted trial balance and adjustment data of Smith Real Estate Appraisal Company at June 30, 2012, follow: Adjustment data at June 30, 2012: a. Prepaid insurance expired, $400. b. Accrued...
-
Turn back to Table 6.3 and consider these three-year strategies: Three-In (fully invested in the risky portfolio in each of the three years); One-In (fully invested in the risky portfolio in one...
-
Identify the likely winners and losers from takeover activity.
-
In November 2010, Angerstein Co. computed its equivalent unit costs under FIFO process costing as follows: Direct material .........$29.50 Packaging ........... 3.00 Direct labor ........... 10.84...
-
14-19 please 14. For Section 351 transfers, immediately after the exchange a. requires simultaneous transfer, if two or more transferors b. allows transfers to occur up to two years apart c. allows...
-
Give the structure of the ester formed when (a) Isobutyric acid reacts with diazomethane in ether. (b) Succinic acid reacts with a large excess of diazomethane in ether. (c) Isobutyric acid reacts...
-
(a) You learned in Sec. 19.7 that carbonyl-addition reactions can occur under basic conditions. The hydrolysis of methyl benzoate is also promoted by OH. Write a mechanism for the hydrolysis of...
-
Which of the following data structure used to implement recursion in the main memory? A. Stack B. Array C. Linked list D. Tree
-
Will the amount of an accrual always be an exact known amount, or could it be an estimate?
-
The reorder point for SKU 303 is 102 units, while average demand during the lead time on an order for SKU 303 is 97 units. How much safety stock is implied by SKU 303's reorder point policy?
-
Find the volume of the solid obtained by rotating the region bounded by the given curves about the specified line. Sketch the region, the solid and a typical disk or washer. -2x 3. y = ex, y = 0, x =...
-
2. Given the list of scores: Score1 = [ 10, 40, 50, 54, 55, 59, 63, 65, 70, 71, 75, 77, 79, 80, 99] The one-sample T-test is used to test whether the mean of Score1 is statistically different from...
-
Find the area of the triangle having the given measurements. Round to the nearest square unit. 13) C=100, a 3 yards, b = 8 yards Use Heron's formula to find the area of the triangle. Round to the...
-
Prove that the pseudoinverse satisfies the following identities: (a) (A+)+ = A (b) A A+A = A (c) A+A A+ = A+ (d) (AA+)T = AA+ (e) (A+A)T = A+A
-
Les has collected stamps in his spare time for years. He purchased many of his stamps at a price much lower than the current market value. Les recently lost his job as a carpenter. Since his wife...
-
The following chlorides (Ph = phenyl) undergo solvolysis in ethanol at the relative rates given in parentheses. How can you explain these results? Ph Ph Ph (3 x 106) (0.08) (300)
-
Provide a detailed mechanism for each of the following reactions. Include contributing resonance structures and the resonance hybrid for the arenium ion intermediates. (a) (b) (c) HNO HSO NO2 Br Bra,...
-
Provide a detailed mechanism for the following reaction. H,SO + H2O
-
Chapter o Homew ebook 50,000-unit production quantity: $ 227,049 7 70,000-unit production quantity: $ 66,751 d. In addition to mean profit, what other factors should FTC consider in determining a...
-
Diamond makes downhill ski equipment. Assume that comic has offered to produce ski poles for Diamond for $20 per pair Diamond needs 200,000 pairs of poles per period Diamond can only avoid 5150,000...
-
17? Which of the following statement is true Select one: a. All evidence must have the same level of reliability b. All evidence must have the same level of persuasiveness C. All are false d....

Study smarter with the SolutionInn App


