(a) You learned in Sec. 19.7 that carbonyl-addition reactions can occur under basic conditions. The hydrolysis of...
Question:
(a) You learned in Sec. 19.7 that carbonyl-addition reactions can occur under basic conditions. The hydrolysis of methyl benzoate is also promoted by –OH. Write a mechanism for the hydrolysis of methyl benzoate in NaOH solution.
(b) A student has suggested the following transformation, arguing that it can be driven to completion with a large excess of methanol and sodium methoxide.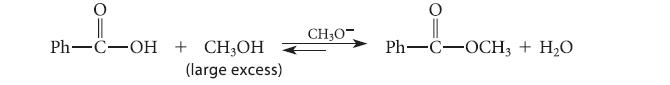
In fact, this reaction does not occur because, under the basic conditions, the carboxylic acid undergoes a different reaction. What is that reaction?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





