The difference in the standard free energies of formation for 1-butene and 2-methylpropene is 13.4 kJ mol
Question:
The difference in the standard free energies of formation for 1-butene and 2-methylpropene is 13.4 kJ mol–1 (3.2 kcal mol–1). (See the previous problem for a definition of ΔG°f .)
(a) Which compound is more stable? Why?
(b) The standard free energy of activation for the hydration of 2-methylpropene is 22.8 kJ mol–1 (5.5 kcal mol–1) less than that for the hydration of 1-butene. Which hydration reaction is faster?
(c) Draw reaction free-energy diagrams on the same scale for the hydration reactions of these two alkenes, showing the relative free energies of both starting materials and rate-determining transition states.
(d) What is the difference in the standard free energies of the transition states for the two hydration reactions?
Which transition state has lower energy? Using the mechanism of the reaction, suggest why it is more stable.
Previous problem
The standard free energy of formation, ΔG°f, is the freeenergy change for the formation of a substance at 25 °C and 1 atm pressure from its elements in their natural states under the same conditions.
Calculate the equilibrium constant for the interconversion of the following alkenes, given the standard free energy of formation of each. Indicate which compound is favored at equilibrium.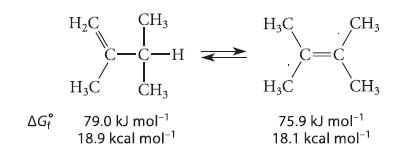
What does the equilibrium constant tell us about the rate at which this interconversion takes place?
Step by Step Answer:






