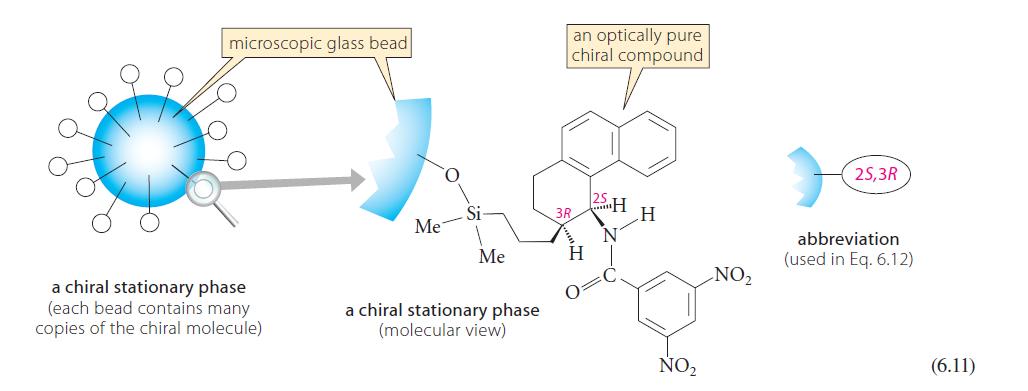
The enantiomeric resolution in Fig. 6.16 used the chiral stationary phase (CSP) in Eq. 6.11. How would
Question:
The enantiomeric resolution in Fig. 6.16 used the chiral stationary phase (CSP) in Eq. 6.11. How would the enantiomeric resolution in Fig. 6.16 be affected if
(a) The enantiomer of the CSP in Eq. 6.11 were used?
(b) The racemate of the CSP in Eq. 6.11 were used?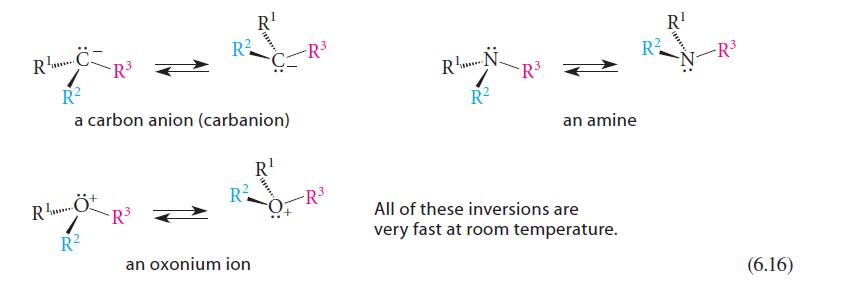
Transcribed Image Text:
CR²³ 3+ R... R² a carbon anion (carbanion) RÖ R² R² -R³ R² R¹ an oxonium ion R¹ -R³ RN R² -R³ R¹ RN-R³² an amine All of these inversions are very fast at room temperature. (6.16)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (4 reviews)
a If the enantiomer of the CSP were used the elution ...View the full answer

Answered By

Ali Khawaja
my expertise are as follows: financial accounting : - journal entries - financial statements including balance sheet, profit & loss account, cash flow statement & statement of changes in equity -consolidated statement of financial position. -ratio analysis -depreciation methods -accounting concepts -understanding and application of all international financial reporting standards (ifrs) -international accounting standards (ias) -etc business analysis : -business strategy -strategic choices -business processes -e-business -e-marketing -project management -finance -hrm financial management : -project appraisal -capital budgeting -net present value (npv) -internal rate of return (irr) -net present value(npv) -payback period -strategic position -strategic choices -information technology -project management -finance -human resource management auditing: -internal audit -external audit -substantive procedures -analytic procedures -designing and assessment of internal controls -developing the flow charts & data flow diagrams -audit reports -engagement letter -materiality economics: -micro -macro -game theory -econometric -mathematical application in economics -empirical macroeconomics -international trade -international political economy -monetary theory and policy -public economics ,business law, and all regarding commerce
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The antitumor drug gimatecan is available as nearly pure (S)-enantiomer. Neither pure (R) enantiomer nor a racemic (equal) mixture of the two enantiomers are available. To measure small quantities of...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
How will the total cost of borrowing be affected if a bond is sold (a) At a discount and (b) At a premium?
-
General-equilibrium effects with labor complementarity. Consider an economy comprised of 100 cities. Each city initially contains 1 million each of high school dropouts, high school graduates,...
-
The Budgetary Comparison Schedule for the City of Salem appears in Illustration 216. Assume the general and subsidiary ledgers for the General Fund were lost after a water pipe burst. You are charged...
-
Management Decision Systems (MDS) is a consulting company that specializes in the development of decision support systems. MDS has a four-person team working on a current project with a small company...
-
What type of an account is the Common Dividend Payable account? AppendixLO1
-
Refer to the Snoey Software Company case. Design a spreadsheet that will determine the annual profit when the prices for the Educational, Large-Scale, and Professional versions are $100, $300, and...
-
Los gerentes deben considerar cul de los siguientes al decidir si subcontratar un producto o servicio? A. Costo cobrado por el producto o servicio B. Calendario de entrega del producto o servicio C....
-
The difference in the standard free energies of formation for 1-butene and 2-methylpropene is 13.4 kJ mol 1 (3.2 kcal mol 1 ). (See the previous problem for a definition of G f .) (a) Which compound...
-
Use the principles of Sec. 1.3B to predict the geometry of BF 3 . What hybridization of boron is suggested by this geometry? Draw an orbital diagram for hybridized boron similar to that for the...
-
Reduction of Order: Second Solution Use the steps o r the formula for y2 developed in Problem 75 to find a second linearly independent solution to the second-order differential equations of Problems...
-
What Is Chemical Energy? Definition and Examples
-
The fundamental concern of computer science is determining what can and cannot be automated. The earliest foundations of what would become computer science predate the invention of the modern digital...
-
History of the United States
-
United States History Pearl Harbor attack
-
Find the Maclaurin polynomial of order 1 for f(x) = x cos x2 and use it to approximate f(0.2)? Discuss.
-
Find the APR in each of the following cases: NUMBER OF TIMES COMPOUNDED Semiannually Monthly Weekly Infinite EAR APR 10.4% 8.9 11.6 15.4
-
How can you account for the fact that 2, 2, 6-trimethylcyclohexanone yields no detectable aldol product even though it has an acidic hydrogen?
-
Cinnamaldehyde, the aromatic constitution of cinnamon oil, can be synthesized by a mixed aldol condensation. Show the starting materials you would use, and write thereaction. CHO Cinnamaldehyde
-
The bicycle ketone shown below does not undergo aldol self-condensation even though it has two ? hydrogen atoms. Explain.
-
When credit terms for a sale are 2/15, n/40, the customer saves by paying early. What percent (rounded) would this savings amount to on an annual basis
-
An industrial robot that is depreciated by the MACRS method has B = $60,000 and a 5-year depreciable life. If the depreciation charge in year 3 is $8,640, the salvage value that was used in the...
-
What determines a firm's beta? Should firm management make changes to its beta? Be sure to consider the implications for the firm's investors using CAPM.

Study smarter with the SolutionInn App


