Use the principles of Sec. 1.3B to predict the geometry of BF 3 . What hybridization of
Question:
Use the principles of Sec. 1.3B to predict the geometry of BF3. What hybridization of boron is suggested by this geometry? Draw an orbital diagram for hybridized boron similar to that for the carbons in ethylene shown in Fig. 4.3, and provide a hybrid orbital description of the bonding in BF3.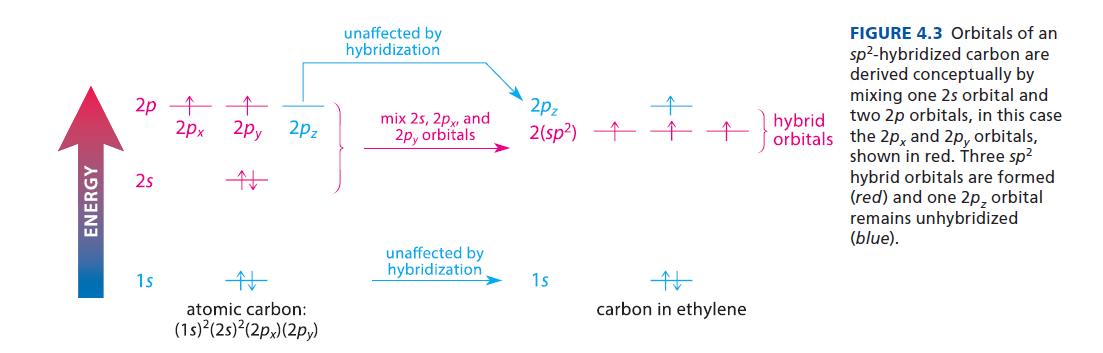
Transcribed Image Text:
ENERGY 2p ↑ 2s ↑ 2px 2py ↑ 1s 2Pz atomic carbon: (1s)²(2s)²(2px) (2py) unaffected by hybridization mix 2s, 2px, and 2p, orbitals unaffected by hybridization 2pz 2(sp²) ↑ 1s # carbon in ethylene FIGURE 4.3 Orbitals of an sp²-hybridized carbon are derived conceptually by mixing one 2s orbital and hybrid two 2p orbitals, in this case orbitals the 2px and 2p, orbitals, shown in red. Three sp² hybrid orbitals are formed (red) and one 2p, orbital remains unhybridized (blue).
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 57% (7 reviews)
Boron trifluoride trifluoroborane BF 3 is trigonal planar with FBF bond angles of 120 Because hybrid...View the full answer

Answered By

Pushpinder Singh
Currently, I am PhD scholar with Indian Statistical problem, working in applied statistics and real life data problems. I have done several projects in Statistics especially Time Series data analysis, Regression Techniques.
I am Master in Statistics from Indian Institute of Technology, Kanpur.
I have been teaching students for various University entrance exams and passing grades in Graduation and Post-Graduation.I have expertise in solving problems in Statistics for more than 2 years now.I am a subject expert in Statistics with Assignmentpedia.com.
4.40+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Use the polygon-and-circle method to draw an orbital diagram for each of the following compounds. (a) (b)
-
The central carbon atom of an allene is a member of two double bonds, and it has an interesting orbital arrangement that holds the two ends of the molecule at right angles to each other. (a) Draw an...
-
5. (12 points total) Let A = Rmxn be a full rank matrix with m n. Such matrices were the object of focus at the end of Unit 3, when we solved least-squares problems. In that unit, we introduced two...
-
Calculate the half life potential for Zn electrode is 0.01M Zn(NO 3 ) 2. Given that Zn 2+ + 2e= Zn E o = 0.763
-
Presented here are several transactions and events of the General Fund of Johnson County. All transactions and events relate to calendar year 2012. 1. Estimated revenues from the following sources...
-
To serve "to-go" orders, Terrapin Coffeehouse faces normally distributed weekly demand with an average of 300 paper cups and a standard deviation of 75 cups per week. Terrapin orders cups by the box....
-
What three crucial dates are involved in the process of paying a cash dividend? AppendixLO1
-
On January 1, 2015, Lennon Corporation acquires 100% of Ono Inc. for $220,000 in cash. The condensed balance sheets of the two corporations immediately following the acquisition are shown below....
-
The price of a stock is $34. A trader buys 1 put option contract on the stock with a strike price of $30 when the option price is $10. When does the trader make a profit? Answer text Question 7
-
The enantiomeric resolution in Fig. 6.16 used the chiral stationary phase (CSP) in Eq. 6.11. How would the enantiomeric resolution in Fig. 6.16 be affected if (a) The enantiomer of the CSP in Eq....
-
Malonic acid has two carboxylic acid groups and consequently undergoes two ionization reactions. The pK a for the first ionization of malonic acid is 2.86; the pK a for the second is 5.70. The pK a...
-
For a constant a > 0, random variables X and Y have joint PDF Find the CDF and PDF of random variable Is it possible to observe W 1/a 0, y a, 0 otherwise. fx,Y (x, y) = XY W=max ( FFT Y,X
-
United States Historyassassination of Martin Luther King, Jr.
-
United States History-Burr-Hamilton duel duel, Weehawken, New Jersey, United States [1804]
-
United States HistoryBattle of Gettysburg American Civil War [1863] When and where was the Battle of Gettysburg fought?
-
United States History - United States presidential election of 1968 United States government
-
Salem witch trials American history What caused the Salem witch trials? How many people were killed during the Salem witch trials?
-
Find the Maclaurin polynomial of order 1 for f(x) = x cos x2 and use it to approximate f(0.2)?
-
Which of the ocean zones shown would be home to each of the following organisms: lobster, coral, mussel, porpoise, and dragonfish? For those organisms you identify as living in the pelagic...
-
What condensation product would you expect to obtain by treatment of the following substances with sodium ethoxide in ethanol? (a) Ethyl Butanoate (b) Cyclopentanone (c) 3, 7-Nonanedione (d)...
-
In the mixed Claisen reaction of cyclopentanone with ethyl format, a much higher yield of the desired product is obtained by first mixing the two carbonyl components and then adding base, rather than...
-
Give the structures of the possible Claisen condensation products from the following reactions. Tell which, if any, you would expect to predominate in each case. (a) CH3CO2Et + CH3CH2CO2Et (b)...
-
Los datos de la columna C tienen caracteres no imprimibles antes y despus de los datos contenidos en cada celda. En la celda G2, ingrese una frmula para eliminar cualquier carcter no imprimible de la...
-
Explain impacts of changing FIFO method to weighted average method in inventory cost valuations? Explain impacts of changing Weighted average method to FIFO method in inventory cost valuations?...
-
A perpetuity makes payments starting five years from today. The first payment is 1000 and each payment thereafter increases by k (in %) (which is less than the effective annual interest rate) per...

Study smarter with the SolutionInn App


