The following compound is unknown, but you are contemplating its synthesis and characterization. Predict its NMR spectrum
Question:
The following compound is unknown, but you are contemplating its synthesis and characterization. Predict its NMR spectrum under each of the following assumptions: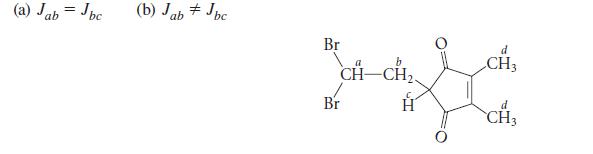
Transcribed Image Text:
(a) Jab = Jbc (b) Jab Jbc. Br b CH-CH₂ d CH3 Lich d CH3 Br
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
a b The resonance for protons He will be a singlet The resonances for protons H Hand He will al...View the full answer

Answered By

Asim farooq
I have done MS finance and expertise in the field of Accounting, finance, cost accounting, security analysis and portfolio management and management, MS office is at my fingertips, I want my client to take advantage of my practical knowledge. I have been mentoring my client on a freelancer website from last two years, Currently I am working in Telecom company as a financial analyst and before that working as an accountant with Pepsi for one year. I also join a nonprofit organization as a finance assistant to my job duties are making payment to client after tax calculation, I have started my professional career from teaching I was teaching to a master's level student for two years in the evening.
My Expert Service
Financial accounting, Financial management, Cost accounting, Human resource management, Business communication and report writing. Financial accounting : • Journal entries • Financial statements including balance sheet, Profit & Loss account, Cash flow statement • Adjustment entries • Ratio analysis • Accounting concepts • Single entry accounting • Double entry accounting • Bills of exchange • Bank reconciliation statements Cost accounting : • Budgeting • Job order costing • Process costing • Cost of goods sold Financial management : • Capital budgeting • Net Present Value (NPV) • Internal Rate of Return (IRR) • Payback period • Discounted cash flows • Financial analysis • Capital assets pricing model • Simple interest, Compound interest & annuities
4.40+
65+ Reviews
86+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The following compound is known to be chiral. Draw its enantiomer, and explain the source of chirality. CH
-
The mass spectrum of unknown compound A shows a molecular ion at m/z 116 and prominent peaks at m/z 87 and m/z 101. Its UV spectrum shows no maximum above 200 nm. The IR and NMR spectra of A follow....
-
Compound A has molecular formula C 8 H 8 O. An IR spectrum of compound A exhibits a signal at 1680 cm -1 . The 1 H NMR spectrum of compound A exhibits a group of signals between 7.5 and 8 ppm (with a...
-
A $250 suit is on sale for $190, and a $90 pair of shoes is on sale for $65. Find the average percent decrease in price for the 2 items.
-
Relating market value to book value of shareholders' equity Firms prepare their balance sheets using authoritative guidance for the recognition and measurement of assets and liabilities. Accountants...
-
Based on Carino and Lenoir (1988). Brady Corporation produces cabinets. Each week, Brady requires 90,000 cubic feet of processed lumber. The company can obtain lumber in two ways. First, it can...
-
Can any of the events in Exercises 5962 be considered unusual? Explain.
-
In Munich a bratwurst costs 5 euros; a hot dog costs $4 at Bostons Fenway Park. At an exchange rate of $1.05/per euro, what is the price of a bratwurst in terms of a hot dog? All else equal, how does...
-
[The following information applies to the questions displayed below.] Raleigh Department Store uses the conventional retail method for the year ended December 31, 2019. Available information follows:...
-
When 3-bromopropene is allowed to react with HBr in the presence of peroxides, a compound A is formed that has the following NMR spectrum: 3.60 (4H, t, J = 6 Hz); 2.38 (2H, quintet, J = 6 Hz). (a)...
-
Give the structure of a compound C 7 H 16 O 3 with the following NMR spectrum: 1.30 (3H, s); 1.93 (2H, t, J = 7.3 Hz); 3.18 (6H, s); 3.33 (3H, s); 3.43 (2H, t, J = 7.3 Hz). Its IR spectrum shows...
-
Flight times, in minutes, were measured for a sample of drones delivering packages. The following display from a TI-84 Plus calculator presents a 99% confidence interval for the population mean...
-
Your introduction needs to include the following. o Include a clear definition of unemployment and inflation and how and why they occur and rise in the economy. o Briefly provide your understanding...
-
Questions: 1. What strategies can be employed to foster a sense of inclusion and belonging within teams, and what are the potential benefits of doing so? 2. How can a team be successful? 3. What is...
-
Critical reflection involves closely examining events and experiences from different perspectives to inform future practice. In a few paragraphs, explain - Why educators should regularly reflect on...
-
What resources does the school or school district provide to teachers to promote diversity, equity, and inclusion? What are some of the strengths and shortcomings of the school's policies on...
-
Select FOUR companies listed on the UK Stock Exchange. Chose two companies from one industry sector and two other companies from another industry sector. By using the most recent three years'...
-
Wasson Company purchased items of inventory as follows: Dec. 2 50 units @ $20 Dec. 12 12 units @ $21 Wasson sold 15 units on December 20. Determine the cost of goods sold for the month under the LIFO...
-
An 8.0 kg crate is pulled 5.0 m up a 30 incline by a rope angled 18 above the incline. The tension in the rope is 120 N, and the crates coefficient of kinetic friction on the incline is 0.25. a. How...
-
What is dn for each of the following complexes? Pd(PPh3)4
-
Within set, rank the compounds in order of increasing rates of their SN2 reactions. Explain your reasoning. 1-bromocyclohexene, bromocyclohexane, 1-(bromomethyl)cyclohexene
-
How many CO ligands would be accommodated by Fe(O) if we assume that the resulting complex follows the 18-electron rule?
-
Comfort Golf Products is considering whether to upgrade its equipment Managers are considering two options. Equipment manufactured by Stenback Inc. costs $1,000,000 and will last five years and have...
-
Weaver Corporation had the following stock issued and outstanding at January 1, Year 1: 71,000 shares of $10 par common stock. 8,500 shares of $60 par, 6 percent, noncumulative preferred stock. On...
-
Read the following case and then answer questions On 1 January 2016 a company purchased a machine at a cost of $3,000. Its useful life is estimated to be 10 years and then it has a residual value of...

Study smarter with the SolutionInn App


