The NMR spectrum of vitamin D 3 is given in Fig. P13.58. Interpret the resonances marked with
Question:
The NMR spectrum of vitamin D3 is given in Fig. P13.58.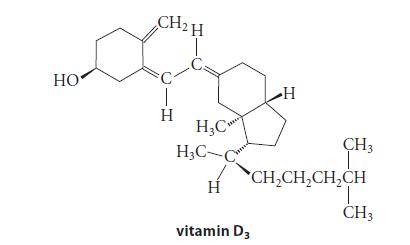
Interpret the resonances marked with asterisks (*) by indicating the part of the structure to which they correspond.
(Do not try to assign the individual resonances within the groups.) Explain your choices.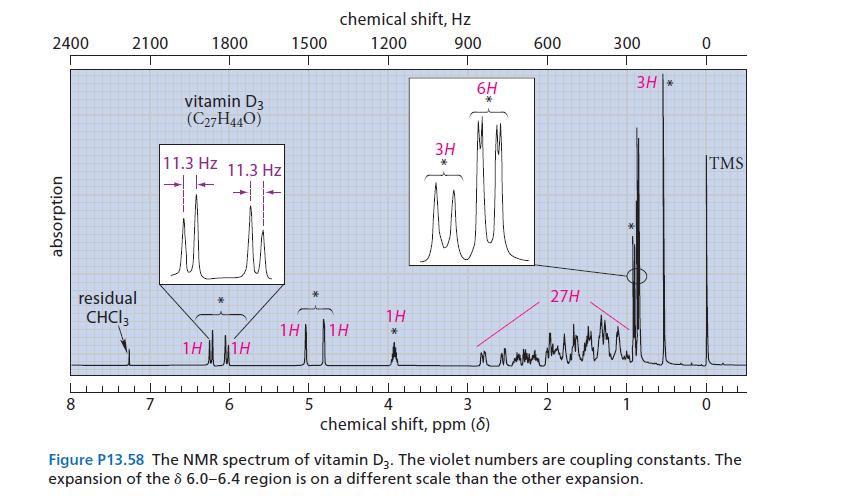
Transcribed Image Text:
HO CH₂H H H₂C H₂C- H H vitamin D3 CH3 CH₂CH₂CH₂CH T CH 3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
The relevant protons a...View the full answer

Answered By

Mehwish Aziz
What I have learnt in my 8 years experience of tutoring is that you really need to have a friendly relationship with your students so they can come to you with their queries without any hesitation. I am quite hardworking and I have strong work ethics. Since I had never been one of those who always top in the class and always get A* no matter what, I can understand the fear of failure and can relate with my students at so many levels. I had always been one of those who had to work really hard to get decent grades. I am forever grateful to some of the amazing teachers that I have had who made learning one, and owing to whom I was able to get some extraordinary grades and get into one of the most prestigious universities of the country. Inspired by those same teachers, I am to be like one of them - who never gives up on her students and always believe in them!
5.00+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The NMR spectrum of toluene (methylbenzene) was shown in Figure 13-11. (a) How many different kinds of protons are there in toluene? (b) Explain why the aromatic region around 7.2 is broad, with...
-
The NMR spectrum of cinnamaldehyde follows. (a) Determine the chemical shifts of Ha, Hb and Hc. The absorption of one of these protons is difficult to see; look carefully at the integrals. (b)...
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
2) Assume that your widget manufacturing company has a total annual demand of N widgets per year evenly distributed across the year. Each widget cost $b dollars in material and manufacturing costs to...
-
The bank statement of Gear Supplies included a $300 NSF check that one of Gear's customers had written to pay for services that were provided by Gear. Required a. Show the effects of recognizing the...
-
At a certain gas station, 40% of the customers use regular gas (A1), 35% use plus gas (A2), and 25% use premium (A3). Of those customers using regular gas, only 30% fill their tanks (event B). Of...
-
Adopt the assumptions of Section
-
Rapid Scooters plans to sell a motorized standard scooter for $ 60 and a motorized chrome scooter for $ 75. Rapid Scooters purchases the standard scooter for $ 45 and the chrome scooter for $ 55....
-
HS 1 2 "nke nha nha nha na nha nha nke onye nwe ya . Aka nt 1 0 , 0 0 0 ga - akw gw ego 3 6 , 0 0 0 Ak k ego kachas h r banyere ego nke Jones Temporary Services b 3 1 / 1 2 / 2 0 1 8 . Kwadebe nkwup...
-
You work for a reputable chemical supply house. An angry customer, Fly Ofterhandle, has called, alleging that a sample of 2,5-hexanediol he purchased cannot be the correct compound. As evidence, he...
-
Propose a structure for the compound that has the following spectra: Mass spectrum: m/z = 152, 150 (equal intensity; double molecular ion) NMR: 81.28 (3H, t, J = 7 Hz); 8 3.91 (2H, q, J = 7 Hz); 8...
-
Based on the financial statements, shown on pages 603-604, for McDonald Carpeting Co. (income statement, statement of owner's equity, and balance sheet), prepare the following financial ratios. All...
-
Let f(x) = x+ 3, x20. The inverse of f is Of 1(x)=x - 3 (f (x) = -x-3 f-(x) = x - 3 Of 1(x) = 3 - x
-
Read the articles and please help me to write the whole assignment perfectly including the citations and references (APA Format). Pleaase choose the country and perspective of a particular industry....
-
A light, inextensible cord passes over a frictionless pulley as shown in figure below. One end of the rope is attached to a block, and a force P is applied to the other end. Block A weighs 600 lb and...
-
BASICOT POST DO NOT ASSIST DO NOT POST DO NOT ASSIST DO NOT POST DO NOT ASSIST For filming a physics demonstration about oscillation, an educational video crew attaches a large spring to a very small...
-
Day Mail Order Co. applied the high-low method of cost estimation to customer order data for the first 4 months of the year. What is the estimated variable order-filling cost component per order...
-
Use the Intermediate Value Theorem to prove that x3 + 3x - 2 = 0 has a real solution between 0 and 1.
-
Suppose that a company has 10.000 outstanding shares in the beginning of the year. On April 1st, the company increases its shares by 6.000. On July 1st, the company increases its shares again, but...
-
What products would you expect from the reaction of 1-bromopropane with each of the following? (a) NaNH2 (b) KOC (CH3)3 (c) NaI (d) NaCN (e) NaC CH (f) Mg, then H2O
-
Which reactant in each of the following pairs is more nucleophilic? Explain. (a) NH2 or NH3 (b) H2O or CH3CO2 (c) BF3 or F (d) (CH3)3P or (CH3)3N (e) I or Cl (f) C N or OCH3
-
Propose structures for compounds that fit the following descriptions: (a) An alkyl halide that gives a mixture of three alkenes on E2 reaction (b) An organo halide that will not undergo nucleophilic...
-
When preparing government-wide financial statements, the modified accrual based governments funds are adjusted. Please show the adjustments (in journal entry form with debits and credits) that would...
-
I need help finding the callable price and call value
-
On 31 October 2022, the owner took goods for his son as a birthday gift. The cost price of the goods was R15 000

Study smarter with the SolutionInn App


