Propose a structure for the compound that has the following spectra: Mass spectrum: m/z = 152, 150
Question:
Propose a structure for the compound that has the following spectra: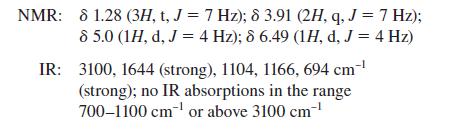
Mass spectrum: m/z = 152, 150 (equal intensity; double molecular ion)
Transcribed Image Text:
NMR: 81.28 (3H, t, J = 7 Hz); 8 3.91 (2H, q, J = 7 Hz); 8 5.0 (1H, d, J = 4 Hz); 8 6.49 (1H, d, J = 4 Hz) IR: 3100, 1644 (strong), 1104, 1166, 694 cm-¹ (strong); no IR absorptions in the range 700-1100 cm¹ or above 3100 cm-¹
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (3 reviews)
The mass spectrum indicates that the compound contains a single bromine double molecular ...View the full answer

Answered By

Ann Wangechi
hey, there, paying attention to detail is one of my strong points, i do my very best combined with passion. i enjoy researching since the net is one of my favorite places to be and to learn. i am a proficient and versatile blog, article academic and research writing i possess excellent English writing skills, great proof-reading. i am a good communicator and always provide feedback in real time. i'm experienced in the writing field, competent in computing, essays, accounting and research work and also as a Database and Systems Administrator
4.90+
151+ Reviews
291+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
A compound with molecular formula C4H8O has a strong IR absorption at 1730 cm-1. Its mass spectrum includes key peaks at m/z 44 (the base peak) and m/z 29. Propose a structure for the compound and...
-
An unknown compound gives a molecular ion of m / z70 in the mass spectrum. It reacts with semicarbazide hydrochloride to give a crystalline derivative, but it gives a negative Tollens test. The NMR...
-
The IR, NMR, and mass spectra are provided for an organic compound. (a) Consider each spectrum individually, and tell what characteristics of the molecule are apparent from that spectrum. (b) Propose...
-
How to find the expected utility of profit under each alternative crop Table 1. Annual Returns to Cropping Alternatives ($ profit / acre) Outcome Worst Bad OK Good Great Probability 0.1 0.2 0.4 0.2...
-
Dick Haney is opening a new business that will sell sporting goods. It will initially be a small operation, and he is concerned about the security of his assets. He will not be able to be at the...
-
An aircraft seam requires 25 rivets. The seam will have to be reworked if any of these rivets is defective. Suppose rivets are defective independently of one another, each with the same probability....
-
Assume the investor has constant relative risk aversion . Define optimal consumption C and terminal wealth WT from the first-order conditions (14.7), and define Wt from (14.5). (a) Show that Wt = M1/...
-
Lansing, Inc., provided the following data for its two producing departments: Machine hours are used to assign the overhead of the Molding Department, and direct labor hours are used to assign the...
-
Suppose you work for a company that sells computers and that you have been asked to oversee doing a physical count and valuation of the inventory at year end. You determine the value of inventory on...
-
The NMR spectrum of vitamin D 3 is given in Fig. P13.58. Interpret the resonances marked with asterisks (*) by indicating the part of the structure to which they correspond. (Do not try to assign the...
-
The two protons H a and H b in 1,2,3-trichloropropane have slightly different chemical shifts, and the splitting pattern of each is a doublet of doublets. For one proton, J 5 9.0 Hz and 4.9 Hz; for...
-
Independent random samples were selected from two normally distributed populations with means m1 and m2, respectively. The sample sizes, means, and variances are LO3 shown in the following table....
-
The four forces, 400, 500, 600 and 700N are acting along the edges of a 0.8m cube as shown. Represent the resultant of these forces by 1) A force Fr through the point A 2) A couple moment Mr (give...
-
Problem 1. What is the degree of freedom of the following mechanism? Sliding joint Sliding joint
-
PILAR Manufacturing Co. has three producing departments (P, I, & L), and two service departments (A&R). The total estimated departmental expenses for 2021 before distribution of service department...
-
1. A volleyball player serves the ball at point A with an initial velocity vo at an angle of 20 to the horizontal. (a) Determine the minimum velocity of the serve such that the ball will just clear...
-
9.50. Dipping low ** A top with I = 3/3 floats in outer space and initially spins around its x3 axis with angular speed w3. You apply a strike at the bottom point, directed into the page, as shown in...
-
A cab company charges $2.50 for the first 1/4 mile and $0.20 for each additional 1/8 mile. Sketch a graph of the cost of a cab ride as a function of the number of miles driven. Discuss the continuity...
-
What are the two components of a company's income tax provision? What does each component represent about a company's income tax provision?
-
Draw all isomers of C4H9Br, name them, and arrange them in order of decreasing reactivity in the SN2 reaction.
-
The following Walden cycle has been carried out. Explain the results, and indicate where Walden inversion isoccurring. OTos H-CH TosCI CHH2 eat CHH2 CHH2 CHCH2 lalp = +33.0 lalp = +31.1 [alp = -19.9...
-
The reactions shown below are unlikely to occur as written. Tell what is wrong with each, and predict the actualproduct. OCCH3)3 . H2CH Br (a) CHCH-CH (CH]l3c (b) Na* - CI LCH3 LCH3 (c) Socil,...
-
What is Coke's average ownership percentage in its equity method investments? Goodwill is 7000 Calculate the firm's current ratio (current assets/current liabilities). Calculate the current ratio...
-
John has to choose between Project A and Project B, which are mutually exclusive. Project A has an initial cost of $30,000 and an internal rate of return of 16 percent. Project B has an initial cost...
-
Complete the table below, for the above transactions

Study smarter with the SolutionInn App


