The IR, NMR, and mass spectra are provided for an organic compound. (a) Consider each spectrum individually,
Question:
The IR, NMR, and mass spectra are provided for an organic compound.
(a) Consider each spectrum individually, and tell what characteristics of the molecule are apparent from that spectrum.
(b) Propose a structure for the compound, and show how your structure fits the spectral data.
(c) Explain why an important signal is missing from the proton NMR spectrum.
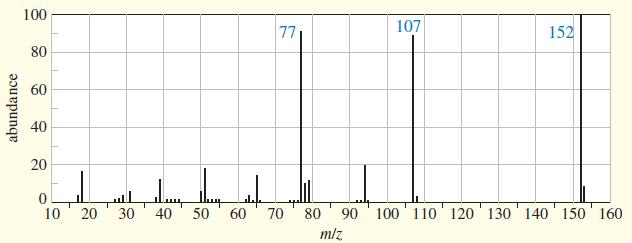
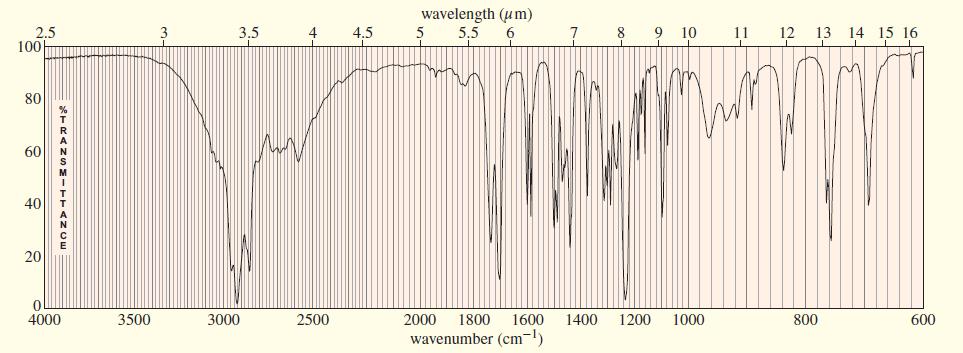
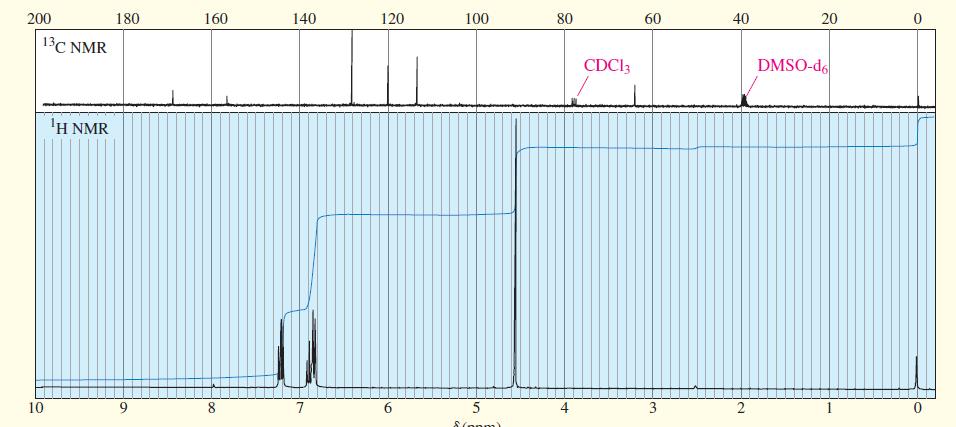
Transcribed Image Text:
100 77 107 152 80 60 40 20 10 20 30 40 50 60 70 80 90 100 110' 120' 130' 140 150 160 miz abundance
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 58% (12 reviews)
a From the mass Spectrum the mz value 152 indicates molecular ion The mz 107 represent the M 45 ...View the full answer

Answered By

Md Muttakin Sarkar
I am now assistant professor at Dumkal College in department of Chemistry. I am also a tuitor in chemistry. I have been teaching chemistry from 2016. Now I am also teaching the students of graduate ,post graduate and different competitive examination. From 5 years of my teaching life ,I am performing this job with grand success.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
An organic compound exhibits IR spectrum F. From the group of structures below, choose one that matches the spectrum best. 0CH3 100 IR 0 4000 3500 3000 2500 2000 1500 1000 600 cm-1 Wavenumber
-
Tell precisely how you would use the proton NMR spectra to distinguish between the following pairs of compounds. (a) 1-bromopropane and 2-bromopropane (b) (c) (d) CH3-CH-t-CH 3 (CH3)2C -_CH3 and CHCH...
-
The IR and 1H NMR spectra for compound X (C8H10) are given in Fig. 14.33. Propose a structure for compound X.
-
Graph the sets of points whose polar coordinates satisfy the equations and inequalitie. 0 /6, r 0
-
BaCl2 H2O(s) loses water when it is heated in an oven: BaCI2 H2O (s) BaCl2(s) + H2O(g) H = 63.11 kJ/mol at 25C S = + 148J(K mol) at 25C (a) Write the equilibrium constant for this reaction....
-
Stock Valuation and PE The Germinating Flower Co. has earnings of $1.75 per share. The benchmark PE for the company is 18. What stock price would you consider appropriate? What if the benchmark PE...
-
Why do you think the media have a negative effect on teen girls dieting habits?
-
Andy, a 10-year-old boy, wants to sell lemonade on a hot summer day. He hopes to make enough money to buy a new iPod. Joe, his elder brother, tries to help him compute his prospect of doing so. The...
-
Q uestion 2: 5 Points Clifford Company is choosing between two projects. The larger project has an initial cost of $100,000, annual cash flows of $30,000 for 5 years. The smaller project has an...
-
The following table details the tasks required for Indiana- based Frank Pianki Industries to manufacture a fully portable industrial vacuum cleaner. The times in the table are in minutes. Demand...
-
The IR, NMR, and mass spectra are provided for an organic compound. (a) Consider each spectrum individually, and tell what characteristics of the molecule are apparent from that spectrum. (b) Propose...
-
Two of the methods for converting alkyl halides to carboxylic acids are covered in Sections 20-8B and 20-8C. One is formation of a Grignard reagent followed by addition of carbon dioxide and then...
-
In the world of work, how do you end up writing a news release in the first place? Likely, the situation is this: your boss has handed you several pages of information and told you to write a news...
-
The adjusted trial balance columns of a worksheet for Levitt Corporation are shown below. The worksheet is prepared for the year ended December 31, Complete the worksheet by (a) entering the adjusted...
-
Derive the commutator $\left[Q_{i}, Q_{j} ight]=i \epsilon_{i j k} Q_{k}$ for the charge defined in Eq. (33.4). Use the charge (33.4) to write the commutator, displaying explicit matrix indices...
-
Verify that the potential $V(\pi, \sigma)$ can be written as Eq. (33.11), and that if $\epsilon=0$ and the symmetry is implemented in the Wigner mode the masses for the $\pi$ and $\sigma$ fields are...
-
Figure 5.7 shows a number of yield curves at various points in time. Go to www.treasury.gov, and in the Resource Center at the top of the page click on Data and Charts Center. Find the Treasury yield...
-
The number of vacation days used by a sample of 20 employees in a recent year In Exercises 2326, use technology to draw a box-and-whisker plot that represents the data set. 3 9 2 17 5 3 2 2 6 4 0 10...
-
Let X and Y be two continuous random variables with joint probability density f(x, y). The joint distribution function F(a, b) is defined as follows: Verify each of the following: a. F (-, -) = F(-,...
-
Read the following description and Write a response of it. The discretion of public administrators can be decreased, but not altogether eliminated. Officials will use their discretion in any given...
-
Draw structures analogous to those in Eqs. 18.59a-d for the catalytic intermediates formed in the conversion of 1, 7-octadiene to cyclohexene and ethylene catalyzed by the G2 catalyst. Eqs. 18.59a-d...
-
Within series, arrange the compounds according to increasing rates of their reactions by the SN1 - E1 mechanism. Explain your reasoning. CH CH Br
-
Which of the, two phenols in each set is more acidic? Explain. Phenol or m-chlorophenol
-
Assignment Title: The Role of Bookkeeping in Business Management and Financial Reporting Objective: Understand the importance of proper bookkeeping procedures in the management of...
-
17) The adjustment that is made to allocate the cost of a building over its expected life is called:A) depreciation expense.B) residual value.C) accumulated depreciation.D) None of the above answers...
-
9) Prepaid Rent is considered to be a(n):A) liability.B) asset.C) contra-asset.D) expense.10) As Prepaid Rent is used, it becomes a(n):A) liability.B) expense. C) contra-asset.D) contra-revenue.11)...

Study smarter with the SolutionInn App


