The two protons H a and H b in 1,2,3-trichloropropane have slightly different chemical shifts, and the
Question:
The two protons Ha and Hb in 1,2,3-trichloropropane have slightly different chemical shifts, and the splitting pattern of each is a doublet of doublets. For one proton, J 5 9.0 Hz and 4.9 Hz; for the other, J 5 9.0 Hz and 6.0 Hz.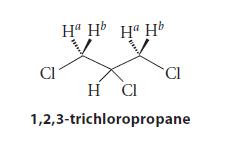
(a) Explain why Ha and Hb have different chemical shifts.
(b) Explain why the splitting pattern for each proton is a doublet of doublets.
Transcribed Image Text:
Cl Ha Hb Ha Hb CI H CI 1,2,3-trichloropropane
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
a Protons a and b are diastereotopic protons and thus give different chemical shifts as dis...View the full answer

Answered By

William Otieno
I am a professional tutor and a writer with excellent skills that are important in serving the bloggers and other specialties that requires a great writer. The important aspects of being the best are that I have served so many clients with excellence
With excellent skills, I have acquired very many recommendations which have made it possible for me to survive as an excellent and cherished writer. Being an excellent content writer am also a reputable IT writer with essential skills that can make one turn papers into excellent result.
4.70+
83+ Reviews
354+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
As the marketing manager for a major snack food producer's salsa and dip division, you know that your product line is most popular with men aged 2 1 to 4 5 . These customers report they typically...
-
The proton NMR spectrum of valine methyl ester hydrochloride is summarized at the top of the next column. Note that protons H e are not split by H d (and vice versa) because protons H e are rapidly...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
Problem 10 The Solow Growth Model is an exogenous model of economic growth that analyzes changes in the level of output in an economy over time as a result of changes in the population growth rate,...
-
Sarah Black worked as the parts manager for Country Automobiles, a local automobile dealership. Sarah was very dedicated and never missed a day of work. Since Country was a small operation, she was...
-
A boiler has five identical relief valves. The probability that any particular valve will open on demand is .96. Assuming independent operation of the valves, calculate P(at least one valve opens)...
-
Assume aggregate consumption C and its expected growth rate satisfy dC C = dt + dB1 d = ( )dt + dB1 + 1 2 dB2 for constants , , , , and and independent Brownian motions B1 and B2. Then, the...
-
The gears are subjected to the couple moments shown. Determine the magnitude and coordinate direction angles of the resultant couple moment. Given: M1 = 40 lb⋅ ft M2 = 30 lb⋅ ft θ1 =...
-
BMX Company has one employee. FICA Social Security taxes are 6.2% of the first $137,700 paid to its employee, and FICA Medicare taxes are 1.45% of gross pay. For BMX, its FUTA taxes are 0.6% and SUTA...
-
Propose a structure for the compound that has the following spectra: Mass spectrum: m/z = 152, 150 (equal intensity; double molecular ion) NMR: 81.28 (3H, t, J = 7 Hz); 8 3.91 (2H, q, J = 7 Hz); 8...
-
To which of the following compounds does the following 13 C NMR-DEPT spectrum belong (attached protons in parentheses): 8 15.5 (3), 8 20.1 (3), 8 60.7 (2), 8 99.6 (1) CH3CH0-CH-OCHCH3...
-
In Question 1, if Jackson acquired the asset on January 1, 1999, and uses the sum-of-the-years'-digits method, the depreciation expense for 1999 is: a. \(\$ 72,727\). b. \(\$ 80,000\). c. \(\$...
-
Describe in your own words how you would expect the data points on a scatterplot to be distributed if the following features were present (i.e. for each part, explain how the feature would look on a...
-
imagine this experimental setup: One temperature probe is in embedded in a small block of frozen sugar water at -20. The frozen sugar water is in a small test tube The melting/freezing point of this...
-
Question 2: (40 points: 10 each) During September, Sweet Foods manufactures a single product. The Company's material purchases amounted to 9,000 pounds at a price of $9.80 per pound. Actual costs...
-
E12-23 (Algo) (Supplement 12B) Preparing a Statement of Cash Flows, Indirect Method: T-Account Approach [LO 12-S2] Golf Goods Incorporated is a regional and online golf equipment retailer. The...
-
A symmetric compound channel in over bank flow has a main channel with a bottom width of 30 m, side slopes of 1:1, and a flow depth of 3m. The floodplains on either side of the main channel are both...
-
A rental car company charges $20 for one day, allowing up to 200 miles. For each additional 100 miles, or any fraction thereof, the company charges $18. Sketch a graph of the cost for renting a car...
-
Ashlee, Hiroki, Kate, and Albee LLC each own a 25 percent interest in Tally Industries LLC, which generates annual gross receipts of over $10 million. Ashlee, Hiroki, and Kate manage the business,...
-
Order each of the following sets of compounds with respect to SN1reactivity: H3C CH3 C NH2 (a) i CH3CH2CHCH3 CI CH (b) (CH)3CI (CH)Br (CH)3C (c) Br CHCH3 C-Br CBr 3
-
Order each of the following sets of compounds with respect to SN2reactivity: CI CH (a) CH3CH2CHCH3 H CH3CH2CH2CI CH (b) CH CH CH CHCCH2Br CHH CH2Br Br CH (c) CH3CH2CH20CH3 CHCH2CH2Br CHCH2CH20Tos
-
Predict the product and give the stereochemistry resulting from reaction of each of the following nucleophiles with (R)-2-bromooctane: (a) CN (b) CH3CO2 (c) CH3S
-
You are evaluating a new project for the firm you work for, a publicly listed firm. The firm typically finances new projects using the same mix of financing as in its capital structure, but this...
-
state, "The subscription price during a rights offering is normally r; lower ; lower r; higher er; higher than the rights-on price and
-
Arnold inc. is considering a proposal to manufacture high end protein bars used as food supplements by body builders. The project requires an upfront investment into equipment of $1.4 million. This...

Study smarter with the SolutionInn App


