The nucleophilic substitution reaction of sodium 2-bromopropanoate with water and/or OH can occur by both an S
Question:
The nucleophilic substitution reaction of sodium 2-bromopropanoate with water and/or –OH can occur by both an SN2 (intermolecular) mechanism and a mechanism that involves neighboring-group participation.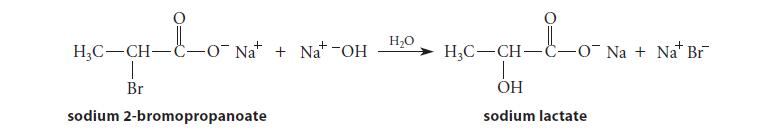
(a) Give the curved-arrow notation for the SN2 mechanism with –OH as the nucleophile.
(b) Give the curved-arrow notation for an intramolecular mechanism. This mechanism should lead you to the structure of an unstable intermediate, which then reacts with –OH to give the product.
(c) The first-order rate constant k1 for the intramolecular reaction is 1.2 * 10–4 s–1. The second-order rate constant for the SN2 reaction is 6.4 * 10–4 M–1 s–1. Calculate the proximity effect for the intramolecular reaction.
(d) At what NaOH concentration does this reaction proceed by the two mechanisms at the same rate?
(e) What is the predominant mechanism in 1 M NaOH?
(f) Consider the structure of the intermediate you derived in part (b). The reason for the small proximity effect is that this intermediate is very unstable. Explain why this intermediate is more unstable than an ordinary epoxide.
Step by Step Answer:






