Use the curved-arrow notation to indicate the flow of electrons in each of the transformations given in
Question:
Use the curved-arrow notation to indicate the flow of electrons in each of the transformations given in Fig. P3.41.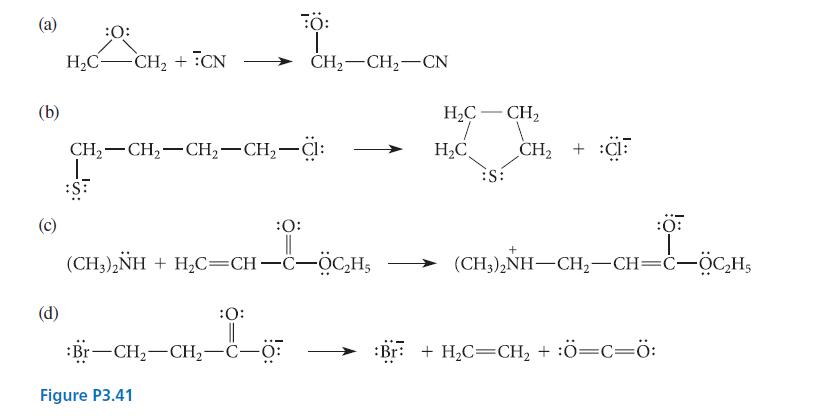
Transcribed Image Text:
(a) (b) (d) :O: H₂C- :S: -CH₂ + CN CH₂ CH₂ CH₂-CH₂-Cl: L FO: :O: || (CH3)2NH + H,C=CH-C–OCH5 OCH :O: || Br-CH₂-CH₂-C-O: Figure P3.41 T CH₂ CH₂ CN H₂C- 7 H₂C - - CH₂ :S: CH₂ + C + HL-5CH (CH,),NH–CH,—CH=c–ỘC,Hs Br + H₂C=CH₂ + :Ö=C=Ö:
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
Remember that an arrow must originate at ...View the full answer

Answered By

Divya Munir
I hold M.Sc and M.Phil degrees in mathematics from CCS University, India and also have a MS degree in information management from Asian institute of technology, Bangkok, Thailand. I have worked at a international school in Bangkok as a IT teacher. Presently, I am working from home as a online Math/Statistics tutor. I have more than 10 years of online tutoring experience. My students have always excelled in their studies.
4.90+
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Use the curved-arrow notation to indicate the flow of electrons in each of the transformations given in Fig. P3.33. (a) (b) (c) (d) (CHJ.NH-CH.-CH-C-OC-H5 BrC2 HC CH CH3 CH2 CH CH C: T:0:
-
Use curved arrow notation to show the bonding changes in the reaction of cis-4-tert-butylcyclohexyl bromide with potassium tert-butoxide. Be sure your drawing correctly represents the spatial...
-
Use curved arrow notation to show the bonding changes in the reaction of cis-4-tert-butylcyclohexyl bromide with potassium tert-butoxide. Be sure your drawing correctly represents the spatial...
-
Considering all of the below, recommend and justify a price for this deal. This is not easy to do. Put yourself in the shoes of the Robertson managers. Who wants it more? The buyer-Monmouth? The...
-
In the 1964 Civil Rights Act, discussed in the text, the bill originally outlawed discrimination based only on race, color, creed, and national origin. Some congressmen supported the addition of sex...
-
The O-H bond lengths in the water molecule (H2O) are 0.96 Ã, and the H-O-H angle is 104.5º. The dipole moment of the water molecule is 1.85 D. (a) In what directions do the bond dipoles...
-
What is the purpose of a project audit? AppendixLO1
-
a. Calculate the centroids for each group in the analysis sample. What do these values indicate? b. Use the regression approach to two-group DA to develop a rule for predicting whether or not a bank...
-
(a) PQS Company Limited prepares its financial statement quarterly. The transaction provided is applicable for the third quarter (July-September). On first August 2021, the company purchased a...
-
In each of the following processes, give the products and classify each of the atoms indicated by a colored label with one or more of the following terms: Brnsted base, Brnsted acid, nucleophilic...
-
Calculate the standard free energy for dissociation of (a) Fluoroacetic acid (pK a = 2.66) (b) Acetic acid (pK a = 4.76)
-
a. Realizing that the most probable outcome from a series of N coin tosses is N/2 heads and N/2 tails, what is the expression for W max corresponding to this outcome? b. Given your answer for part...
-
Most businesses have been impacted negatively in 2020 by the outbreak of Corona virus leading to the disease Covid 19. Many countries went in lock down where by economic activities nearly came to a...
-
The unadjusted trial balance has been entered on a 10-column end-of-period spreadsheet work sheet) for you. Complete the spreadsheet using the following adjustment data a Physcial inventory count on...
-
A) What should be the price of the call option? B) Assume that the call option on Apple with strike price $90 and maturity in one year is currently trading at $17. You immediately tell your broker...
-
White Company has two departments, Cutting and Finishing. The company uses job-order costing and computes a predetermined overhead rate in each department. The Cutting Department bases its rate on...
-
Can someone please help me figure out how to find the qualified business income for this problem? Maria and Javier are the equal partners in MarJa, a partnership that is a qualifying trade or...
-
Distinguish between the ways that mutations in oncogen and tumor suppressor genes cause cancer.
-
2.) Find the Laplace transform of f(t) 7e-St cos 2t +9 sinh2 2t. Use Laplace Table. %3D
-
Approximately how much steric strain does the 1, 3-diaxial interaction between the two methyl groups introduce into the diaxial conformation of cis-1, 3-dirnethylcyclohexanc?
-
In light of your answer to Problem 4.44, draw the two chair conformations of 1, 1, 3-trimcthylcyclohexanc, and estimate the amount of strain energy in each. Which conformation is favored?
-
We saw in Problem 4.20 that cis-decalin is less stable than trans-decalin. Assume that the 1, 3-diaxial interactions in trans-decalin are similar to those m axial methylcyclohexane [that is, one CH2H...
-
3. The nominal interest rate compounded monthly when your $7,000 becomes $11,700 in eight years is ________
-
An investor can design a risky portfolio based on two stocks, A and B. Stock A has an expected return of 21% and a standard deviation of return of 39%. Stock B has an expected return of 14% and a...
-
Advanced Small Business Certifica Drag and Drop the highlighted items into the correct boxes depending on whether they increase or decrease Alex's stock basis. Note your answers- you'll need them for...

Study smarter with the SolutionInn App


