Use the known stereochemistry of the starting alkene (from this section) to assign the stereochemical configuration of
Question:
Use the known stereochemistry of the starting alkene (from this section) to assign the stereochemical configuration of the product, which was found by experiment to be levorotatory.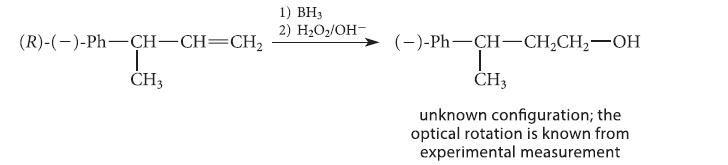
Transcribed Image Text:
(R)-(-)-Ph-CH-CH=CH₂ | CH3 1) BH3 2) H₂O₂/OH- (-)-Ph-CH-CH₂CH₂-OH I CH3 unknown configuration; the optical rotation is known from experimental measurement
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (5 reviews)
Begin with the perspective structure of the reactant in ...View the full answer

Answered By

Carly Cimino
As a tutor, my focus is to help communicate and break down difficult concepts in a way that allows students greater accessibility and comprehension to their course material. I love helping others develop a sense of personal confidence and curiosity, and I'm looking forward to the chance to interact and work with you professionally and better your academic grades.
4.30+
12+ Reviews
21+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Explain how you would use the alkene starting material in Problem 6.13 to determine the absolute configuration of the dextrorotatory enantiomer of the following hydrocarbon: Indicate what reaction...
-
From time to time courts are called on to determine the enforceability of an arbitration clause contained in a contract. One such case was DiMercurio v.Sphere Drake Insurance, 202 F. 3d 71 (1st Cir....
-
Read the case study "Southwest Airlines," found in Part 2 of your textbook. Review the "Guide to Case Analysis" found on pp. CA1 - CA11 of your textbook. (This guide follows the last case in the...
-
Accurate Job Costing must be done on three levels. Which of the following is not one of these levels? Tracking and controlling costs during jobs Tracking gross profit each month Filing records on...
-
Assuming the guards did a bad job assisting the passenger, was the railroad liable for the injuries to Ms. Palsgraff?
-
Write the chemical formula for each substance mentioned in the following word descriptions (use the front inside cover to find the symbols for the elements you don't know). (a) Zinc carbonate can be...
-
Experiment: Choosing a month of the year Event: Choosing a month that begins with the letter J
-
Top management of Drexel-Hall is considering closing Store 3. The three stores are close enough together that management estimates closing Store 3 would cause sales at Store 1 to increase by $60,000,...
-
Gummy Land Candies manufactures jaw-breaker candies in a fully automated process. The machine that produces candies was purchased recently and can make 5,000 per month. The machine costs $6,500 and...
-
Explain why the following compound has two meso stereoisomers. | | | H3C-CH-CH-CH-CH3
-
A chemist has developed a new synthesis of ibuprofen and has reported that she has prepared the (S)-(1)-enantiomer in 90% EE, and that this material has a measured specific rotation of 151.7 degrees...
-
List and describe the steps in the decision-making process.
-
Idenfity whether the following book - tax adjustments are permanent or temporary differences. ( a ) Federal Income Tax Expense ( b ) Depreciation Expense ( c ) Accrued Compensation ( d ) Dividends...
-
2 . ) Pozycki, LLC has reported losses of $ 1 0 0 , 0 0 0 per year since its founding in 2 0 1 6 . For 2 0 2 3 , Pozycki anticipates a profit of about $ 1 0 0 , 0 0 0 . There are 3 equal members of...
-
Elena is a single taxpayer for tax year 2023. On April 1st, 2022, Elena's husband Nathan died. On July 13, 2023, Elena sold the residence that Elena and Nathan had each owed and used as their...
-
Rodriguez Corporation issues 12,000 shares of its common stock for $56,600 cash on February 20. Prepare journal entries to record this event under each of the following separate situations. 1. The...
-
Problem 3: A large rectangular plate is loaded in such a way as to generate the unperturbed (i.e. far-field) stress field xx = Cy; yy = -C x; Oxy = 0 The plate contains a small traction-free circular...
-
Explain the causes of pituitary dwarfism and gigantism.
-
For each of the following transactions, indicate whether it increases, decreases, or has no effect on the following financial ratios: current ratio, debt-to-equity ratio, profit margin ratio, and...
-
Rank the following halides in order of their reactivity in the Williamson synthesis: (a) Bromo ethane, 2-bromopropane, bromo benzene (b) Chloro ethane, bromo ethane, 1-iodopropene
-
Predict the products of the following reactions: (a) (b) CH CH3CH2CH-0-CH2CH2CH3 CH r HBr
-
Write the mechanism of the acid-catalyzed cleavage of tert-butyl cyclohexyl ether to yield Cyclohexanol and 2-methylpropene.
-
Management makes many judgements and estimates in preparing accounts, some of which will have a significant effect on the reported results and financial position. Give examples of ZAIN estimates and...
-
What is the NPV of a project with an initial investment of $350,000 and annual cash inflows of $150,000 for the next 10 years? Cost of capital is 13% A $436,721.21 B $442,901.59 C $452,932.43 D...
-
Journal DATE DESCRIPTION POST. REF. DEBIT CREDIT 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 Joumalize the entries for the following transactions. Refer to the Chart of Accounts for exact wording of...

Study smarter with the SolutionInn App


