Using abbreviated structures like the ones in Eq. 25.9a, give a curved-arrow mechanism for the reduction of
Question:
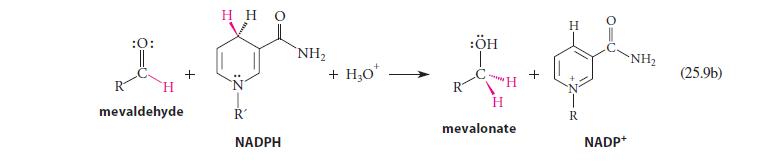
Using abbreviated structures like the ones in Eq. 25.9a, give a curved-arrow mechanism for the reduction of mevaldehyde shown in Eq. 25.9b.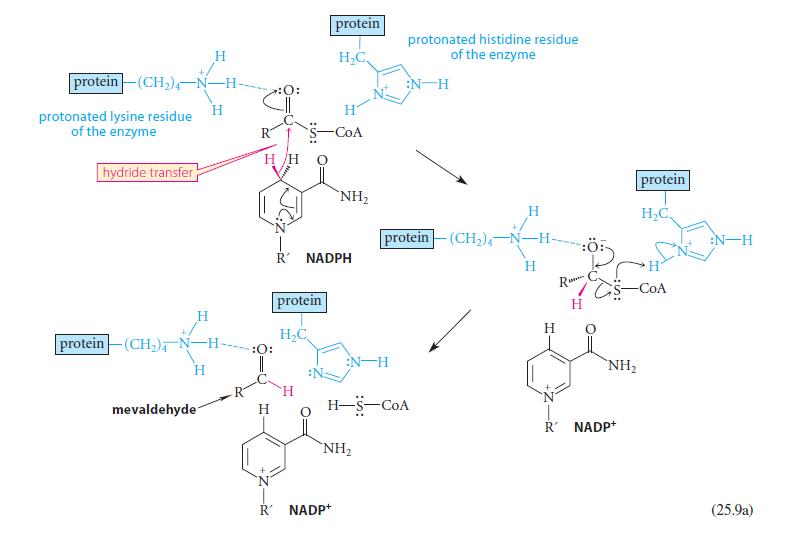
Transcribed Image Text:
protein (CH₂)-N-H- protonated lysine residue of the enzyme hydride transfer H mevaldehyde H H protein (CH₂), N-H- H H/H :O: :O: H protein H₂C H protein H₂C, R NADPH -CoA NH₂ R' NADP+ NH₂ protonated histidine residue of the enzyme :N H protein(CH₂)-N- -H H-S-COA H H H R TG H NH₂ R NADP+ protein H₂C -COA :N-H (25.9a)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
Despite the central phosphorus being tetrahedral with four different gro...View the full answer

Answered By

Monica Malik
I have been teaching Physics and Mathematics for over five years now. I ran a tutorial in Goa where I tutored students between the age of five and eighteen. Earlier this year (2019) I got TEFL certification and now I teach English to students from six countries for two different online portals.
I volunteered in India, Nepal, Srilanka, Thailand and Cambodia and enjoy teaching a lot. I am looking forward to connecting with more students so the learning and teaching can continue.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Using abbreviated structures like the ones used in this section, outline the steps that convert hexanoyl-ACP into octanoyl-ACP during fatty-acid biosynthesis.
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
The hydrolysis of phosphotyrosine esters in proteins is catalyzed by a family of enzymes called protein phosphotyrosine phosphatases. These hydrolyses in many cases involve phosphoenzyme...
-
John dies on 3 March 2021. Between 6 April 2020 and 3 March 2021, he has capital gains of 1,200 and capital losses of 15,400. His net gains in recent tax years (and the annual exemption for each...
-
Using Table 40-1, find the Q values for the following reactions: (a) 1 H + 3 H 3 He + n + Q and (b) 2 H + 2 H 3 He + n + Q Table 40-1 Atomic Masses of the Neutron and Selected Isotopes Element...
-
For what absolute value of the phase angle does a source deliver 75% of the maximum possible power to an RLC circuit?
-
12C6 10C7
-
The following information is available from the accounting records of Eva Corporation: Fixed costs per period are $4800. Sales volume for the last period was $19 360, and variable costs were $13 552....
-
Yahoo! Inc.'s recent financial statements contain the following selected data (in thousands). Current assets Total assets $ 4,594,772 14,936,030 Current liabilities Total liabilities $1,717,728...
-
The following chiral phosphate ester cannot be isolated in optically active form. Explain. O CH3O-P-OH OCHCH3
-
The side chain R of the amino acid serine is CH 2 OH. Draw the structure of the phosphate monoester of serine.
-
Its not the fall that hurts you; its the sudden stop at the bottom. Translate this saying into the language of Newtons laws of motion?
-
Micro-Brush requires a new component for their laptop cleaning machines. The company must decide whether to make or buy them. If it decides to make them. Should it use process A or process B? Use a...
-
Moving from a fee-for-service to a managed care delivery system set up a series of expectations (page 421). How many of these expectations are realistic? How many have been realized?
-
2. A 55 kg human is shot out the end of a cannon with a speed of 18 m/s at an angle of 60. Ignore friction and solve this problem with energy conservation. As he exits the cannon, find: a. horizontal...
-
Theoretical Background: Information Assurance (IA) architecture also known as security architecture is about planning, integrating and continually monitoring the resources of an organization so they...
-
AZCN recommends Microsoft Lens or Adobe Scan; download one of these to yo phone via your phone's app store 2. Place the document you want to scan on a flat, well-lit surface. Make sure the document...
-
A bullet leaves the barrel of a rifle with a speed of 300.0 m/s. The mass of the bullet is 10.0 g. (a) What is the de Broglie wavelength of the bullet? (b) Compare with the diameter of a proton...
-
In Exercises evaluate the limit, using LHpitals Rule if necessary. lim 07x cos x X
-
Outline a Preparation of the following compounds from aniline and any other reagents. Sulfathiazole, a sulfa drug. H2N sulfathiazole
-
Outline a synthesis for each of the following compounds from the indicated starting materi-als using a reaction sequence involving a diazonium salt. (a) 2-bromobenzoic acid from o-toluidine...
-
What two compounds would react in a diazo coupling reaction to form FD & C Yellow No. 6?
-
Hite corporation intends to issue $160,000 of 5% convertible bonds with a conversion price of $40 per share. The company has 40,000 shares of common stock outstanding and expects to earn $600,000...
-
Your portfolio has a beta of 1.17, a standard deviation of 14.3 percent, and an expected return of 12.5 percent. The market return is 11.3 percent and the risk-free rate is 3.1 percent. What is the...
-
Slow Roll Drum Co. is evaluating the extension of credit to a new group of customers. Although these customers will provide $198,000 in additional credit sales, 13 percent are likely to be...

Study smarter with the SolutionInn App


