The following chiral phosphate ester cannot be isolated in optically active form. Explain. O CH3O-P-OH OCHCH3
Question:
The following chiral phosphate ester cannot be isolated in optically active form. Explain.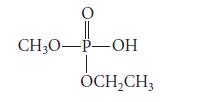
Transcribed Image Text:
O CH3O-P-OH OCH₂CH3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 40% (5 reviews)
Despite the central phosphorus being tetrahedral with four differ...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Determine whether each of the following compounds can be isolated in optically active form. Explain how you know. (a) Trans-1,2-dimethylcyclohexane (b) 1,1-dimethylcyclohexane (c)...
-
Consider a bank branch that has three distinct customer arrival patterns throughout the day, as measured by average arrival rates (below). Morning (8:30-11:30): arrival 1 = 38 per hour. Lunch (11:30...
-
Explain why the quaternary ammonium salt A can be isolated in optically active form, but the trialkylammonium salt B cannot. CH3 T PhCH,N*CH,CH, C1- CHCHCH3 A CH3 PhCH NCHCH3 CI- - 1 H B
-
On what date is CGT for 2020-21 normally due for payment?
-
Using Table 40-1, find the Q values for the following reactions: (a) 2 H + 2 H 3 H + 1H + Q, (b) 2 H + 3 He 4 He + 1 H + Q, (c) 6 Li + n 3 H + 4 He + Q. Table 40-1 Atomic Masses of the Neutron and...
-
a. What is the peak current supplied by the emf in FIGURE P32.45?b. What is the peak voltage across the 3.0 F capacitor? 3.0 F Eo = 10 V f=200 Hz 4.0 F: -2.0 F FIGURE P32.45
-
7P4 SC4
-
Brinkley Company, which began operations on January 3, 2015, had the following subsequent transactions and events in its long-term investments. 2015 Jan. 5 Brinkley purchased 20,000 shares (25% of...
-
The financial statements for Highland Corporation included the following selected information: The common stock was sold at a price of $30 per share. 3. How many shares are in treasury stock
-
The stereochemistry of substitutions in phosphate esters is sometimes studied with compounds in which sulfur is used in place of one oxygen. For example, the F16BP-catalyzed hydrolysis of...
-
Using abbreviated structures like the ones in Eq. 25.9a, give a curved-arrow mechanism for the reduction of mevaldehyde shown in Eq. 25.9b. protein (CH)-N-H- protonated lysine residue of the enzyme...
-
Strategic planning is used to set priorities. Explain why it is important for the company. LO.1
-
What are the key differences between OLTP (Online Transaction Processing) and OLAP (Online Analytical Processing) databases, and how do they cater to distinct business requirements ?
-
__________ refers to speaking up with good intentions about work-related issues, rather than remaining silent. Multiple Choice Neutralizing Micromanagement Filtering Voice Collaborating
-
Consider Michael Porter's Five Forces Model and use the enclosed form to evaluate the OCSIP industry in Jamaica.
-
Petesy Corporation is preparing its Master Budget for 2019. Budget information is as follows: Sales Production Cost Operating Expenses 2019 1 st Quarter P280,000 P192,000 P64,000 2 nd Quarter 320,000...
-
Design a DFA to recognize any valid fractional numbers of the form . where is at most 3 digits and is any number of digits. However, fractional part can never have more digits than the wholepart. If...
-
A beam of neutrons is used to study molecular structure through a series of diffraction experiments. A beam of neutrons with a wide range of de Broglie wavelengths comes from the core of a nuclear...
-
What is the shape of the exponential distribution?
-
What product (including its stereochemistry) is expected from the Hofmann elimination of each of the following stereoisomers? N(CH3)3 OH (2R,3S)- Ph CH CH Ph CHy
-
Give an acceptable name for the following compounds. (a) (b) CH3 CH3 NH
-
Given the major product formed when each of the following amines is treated exhaustively with methyI iodide and then heated with Ag2O.Explain your reasoning. Ph N.
-
In the context of portfolio theory, what is diversification primarily intended to do ? A ) Increase returns. B ) Reduce risk. C ) Maximize tax efficiency. D ) Simplify investment management.
-
4. The risk-free rate of return is 3.78% and the market risk premium is 6.42%. What is the expected rate of return on a stock with a beta of 1.09?
-
Maddox Resources has credit sales of $ 1 8 0 , 0 0 0 yearly with credit terms of net 3 0 days, which is also the average collection period. Maddox does not offer a discount for early payment, so its...

Study smarter with the SolutionInn App


