The stereochemistry of substitutions in phosphate esters is sometimes studied with compounds in which sulfur is used
Question:
The stereochemistry of substitutions in phosphate esters is sometimes studied with compounds in which sulfur is used in place of one oxygen. For example, the F16BP-catalyzed hydrolysis of fructose-1,6-bisphosphate in H217O was studied with the following sulfur-substituted analog: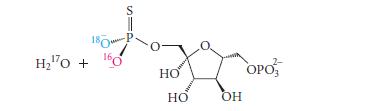
a. Why is the substitution of sulfur for oxygen important when studying the stereochemistry of this reaction?
b. Assuming that the substitution of sulfur for oxygen does not change the mechanism of the reaction, what is the product of this reaction and what is its stereochemistry?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





