Using the mechanism of the oxymercuration reaction to guide you, predict the product(s) obtained when 1-hexene is
Question:
Using the mechanism of the oxymercuration reaction to guide you, predict the product(s) obtained when 1-hexene is treated with mercuric acetate in each of the following solvents and the resulting products are treated with NaBH4/NaOH. Explain your answers and tell what functional groups are present in each of the products.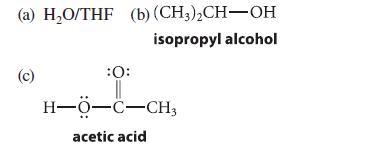
Transcribed Image Text:
(a) H₂O/THF (b) (CH3)2CH-OH isopropyl alcohol (c) :0: || H−O−C−CH, acetic acid
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
The reasoning is similar to that used in the solution to Problem 5...View the full answer

Answered By

Prerit Goyal
Education:
I am a postgraduate in Mathematics with 77.90% from University of Kota, Rajasthan, India.
I have completed my graduation in BSc(H) Mathematics with 84.70% from University of Delhi, India.
I had an excellent academic record throughout my studies. And now I want to share my knowledge with the students seeking help in solving the Mathematics problems.
I usually focus on the concept of a problem, so that it becomes easier to solve similar other problems.
Tutoring experience:
I have worked as a Subject Matter Expert on various reputed platforms.
I try to provide easy to understand solutions to the students in clear handwriting, making them aware about the concept used and also the mistake prone areas.
It feels great when a student is able to get the solution and appreciates the efforts for providing the solution.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
When (3s,4S)-4-methoxy-3-methyl- I -pentene is treated with mercuric acetate in methanol solvent, then with NaBH4, two isomeric compounds with the formula C8H18O2, are isolated. One, compound A, is...
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
List three specific parts of the Case Guide, Objectives and Strategy Section (See below) that you had the most difficulty understanding. Describe your current understanding of these parts. Provide...
-
Can HRM specialists rise to the challenge of promoting standardisation while also remaining the guardians of national culture in an organisation?
-
Zion Manufacturing had always made its components in-house. However, Bryce Component Works had recently offered to supply one component, K2, at a price of $25 each. Zion uses 10,000 units of...
-
Some hotels believe that self check-in and self check-out by guests will be appealing because it will save the guest time. Other hoteliers believe that guests desire contact with hotel staff during...
-
When I get behind in my payments, I ignore the past-due notices. (A) Never or not applicable (B) Sometimes (C) Always
-
Ken, a salaried employee, was terminated from his company in April of this year. Business had been slow since the beginning of the year, and each of the operating plants had laid off workers. Ken's...
-
A taxpayer can acquire property for (1) use in a business, (2) for resale by a business, or (3) as investments. For each of these three situations indicate (1) How each of the properties would be...
-
Using the mechanism of halogen addition to alkenes to guide you, predict the product(s) obtained when 2-methyl-1-butene is subjected to each of the following conditions. Explain your answers. (a) Br...
-
Repeat Problem 5.27 for 1-ethylcyclopentene. Problem 5.27 Give the principal organic products expected when 1-butene reacts with each of the following reagents. (a) Br in CHCl solvent (b) 03, -78 C...
-
If your Competitive Profile Matrix has three firms and they all end up with the same Total Weighted Score, would the analysis still be useful? Why.
-
Using the ideas of kinetic particle theory when you come home from school and open the door you can smell food being cooked
-
The following information relates to Salamat Corporation for the last year.Salamat uses direct labor hours as an overhead base. Estimated direct labor hours 360,000 hours Estimated manufacturing...
-
Code in matlab the translational motion via numeric integration of the orbit (two-body orbit sufficient). Use the orbital characteristics of the Centaur V upper stage from the Atlas V launch on...
-
Lolita Company has the following information available for June 2020: Beginning Work in Process Inventory (25% as to conversion) 20,000 units Started 130,000 units Ending Work in Process Inventory...
-
Question 3 (15 marks) Sporty Ltd. produces scooters and skateboards. At the beginning of the year, the following volume of activities were budgeted for the year: Production volume/units Direct labour...
-
Based on your answer to Problem 1-12, categorize each of the following conclusions as being the result of positive analysis or normative analysis. a. A higher minimum wage will reduce employment...
-
1. Which of the four major types of information systems do you think is the most valuable to an organization? 2. How do you critically associate the ideas of business agility and business efficiency...
-
Explain which compound is the stronger acid: (a) CHF2CO2HorCH2FCO2H (b) CHF2CO2HorCHBr2CO2H (c) CH3OCH2CO2HorCH3CO2H
-
Which is the most acidic hydrogen in CH3CH2C = CH?
-
Show the resonance structures for the conjugate base of the Meta isomer of nitro-phenol and confirm that the nitro group is less effective at stabilizing this anion than it is in the case of the Para...
-
Famas Llamas has a weighted average cost of capital of 8.8 percent. The companys cost of equity is 12 percent, and its pretax cost of debt is 6.8 percent. The tax rate is 22 percent. What is the...
-
The common stock of a company paid 1.32 in dividens last year. Dividens are expected to gros at an 8 percent annual rate for an indefinite number of years. A) If the company's current market price is...
-
(1 point) Bill makes annual deposits of $1900 to an an IRA earning 5% compounded annually for 14 years. At the end of the 14 years Bil retires. a) What was the value of his IRA at the end of 14...

Study smarter with the SolutionInn App


