What do the pericyclic selection rules have to say about the position of equilibrium in each of
Question:
What do the pericyclic selection rules have to say about the position of equilibrium in each of the reactions given in Fig. P28.30? Which side of each equilibrium is favored and why?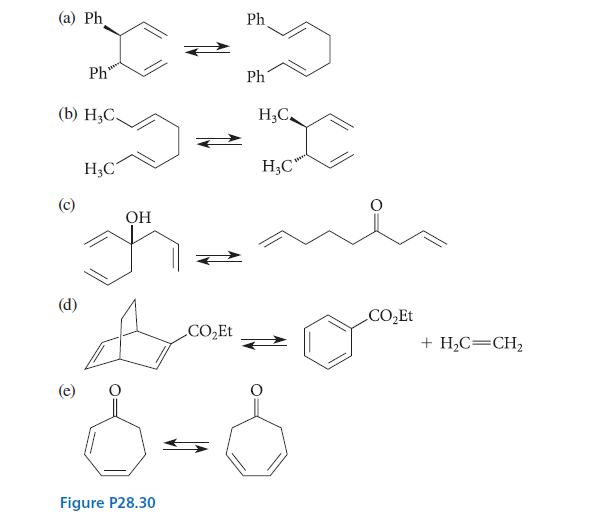
Transcribed Image Text:
(a) Ph (b) H₂C. (c) Ph (d) H₂C OH Ph Ph H₂C. H₂C" CO₂Et CO₂Et Bom Do "8-8 Figure P28.30 + H₂C=CH₂
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
The pericyclic selection rules say absolutely nothing about the position of equilibrium in each case ...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
What do the pericyclic selection rules have to say about the position of equilibrium in each of the reactions given in Fig. P27.30? Which side of each equilibrium is favored and why? Fig. P27.30 (a)...
-
Goals of this unit: 1. construct cogent, logical, effective, and ethical arguments in writing; 2. compose texts that effectively employ the features of a given genre; 3. identify reliable and...
-
1) What is the definition that the authors use for the concept of strategic ambidexterity? 2) What do the authors have to say about organizational capabilities and exploitive strategies? 3) What do...
-
The CFO of the Jordan Microscope Corporation intentionally misclassified a downstream transportation expense in the amount of $575,000 as a product cost in an accounting period when the company made...
-
FedEx is the worlds leading express-distribution company. In addition to the worlds largest fleet of allcargo aircraft, the company has more than 654 aircraft and 51,000 vehicles and trailers that...
-
If the product is an extension of one in your current product mix, determine the type(s) of modifications that will be performed.
-
From the following particulars, calculate (a) SVV, (b) SPV and (c) Sales volume variance. The budgeted and actual sales for a period in respect of two products are as follows: Product Budgeted...
-
Cabot Appliances, a retail chain, is trying to decide what size order it should place with its supplier of room air conditioners. Room air conditioner sales are highly seasonal, and the number of...
-
Quality Kitchen Companys 2021 single-step income statement and comparative balance sheet are provided below: QUALITY KITCHEN COMPANY Income Statement Year Ended December 31, 2021...
-
Heptafulvalene undergoes a thermal reaction with tetracyanoethylene (TCNE) to give the adduct shown in Fig. P28.36. What is the stereochemistry of this adduct? Explain. heptafulvalene Figure P28.36...
-
When previtamin D 2 (which is identical to previtamin D 3 , except for the R-group) is isolated and irradiated, ergosterol is obtained along with a stereoisomer, lumi sterol. Explain mechanistically...
-
Adobe Systems is well known as the maker of Acrobat, Photoshop, Flash, and other programs that are fundamental tools in the Internet Age. It is also becoming well known as one of the "greenest"...
-
Sample for a Poll There are 30,488,983 Californians aged 18 or older. If The Gallup organization randomly selects 1068 adults without replacement, are the selections independent or dependent? If the...
-
Part A: You have successfully graduated Conestoga College and have joined a public accounting firm in their tax department. You have been assigned to work on a project with Emily Wilson, one of the...
-
Write a program that gets a list of integers from input, and outputs negative integers in descending order (highest to lowest). Ex: If the input is: 10 -7 4-39 -6 12 -2 the output is: -2-6-7-39 For...
-
The manager of a division that produces add-on products for the automobile industry had just been presented the opportunity to invest in two independent projects. The first is an air conditioner for...
-
4. We are interested in the effect on test scores of the student-teacher ratio (STR). The following regression results have been obtained using the California data set. All the regressions used...
-
Romano's Frozen Pizza Inc. has determined from its production budget the following estimated production volumes for 12" and 16" frozen pizzas for September 2016: There are three direct materials used...
-
Read Case Study Google: Dont Be Evil Unless and answer the following: Given its mission of providing information to the world, should Google censor searches in China?
-
The percent s character describes the hybridization of an orbital. For example, an ,sp3 orbital has 25%s character. Given the bond angles in each case, calculate the percent s character of (1) the...
-
Consider the resonance structures for the carbonate ion. (a) How much negative charge is on each oxygen of the carbonate ion? (b) What is the bond order of each carbon-oxygen bond in the carbonate...
-
Orbitals with l : 3 are called"f orbitals. (a) How many energetically equivalent f orbitals are there? (b) In [vhat principal quantum level do f orbitals flrst appear? (c) How many nodes does a 5f...
-
Slow Roll Drum Co. is evaluating the extension of credit to a new group of customers. Although these customers will provide $198,000 in additional credit sales, 13 percent are likely to be...
-
Wendell's Donut Shoppe is investigating the purchase of a new $39,600 conut-making machine. The new machine would permit the company to reduce the amount of part-time help needed, at a cost savings...
-
1.Discuss the challenges faced with Valuing Stocks and Bonds. As part of this discussion, how will the selected item be implemented in an organization and its significance? 2. Discuss how Valuing...

Study smarter with the SolutionInn App


