When 1,3,5-cyclooctatriene, A, is heated to 80100C, it comes to equilibrium with an isomeric compound B. Treatment
Question:
When 1,3,5-cyclooctatriene, A, is heated to 80–100°C, it comes to equilibrium with an isomeric compound B. Treatment of the mixture of A and B with CH3O2C—C≡C—CO2CH3 gives a compound C, which, when heated to 200°C for 20 minutes, gives dimethyl phthalate and cyclobutene. Identify compounds B and C, and explain what reactions have occurred.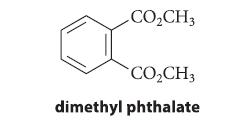
Transcribed Image Text:
CO₂CH3 CO₂CH3 dimethyl phthalate
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (5 reviews)
Take a cue from the solution to Problem 2831a on p 1031XR o...View the full answer

Answered By

Loise Ndungu
I have five years of experience as a writer. As I embark on writing your papers from the prologue to the epilogue, my enthusiasm is driven by the importance of producing a quality product. I put premium product delivery as my top priority, as this is what my clients are seeking and what makes me different from other writers. My goal is to craft a masterpiece each time I embark on a freelance work task! I'm a freelance writer who provides his customers with outstanding and remarkable custom writings on various subjects. Let's work together for perfect grades.
4.90+
82+ Reviews
236+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
A compound A (C 11 H 14 O 3 ) is insoluble in base and gives an isomeric compound B when heated strongly. Compound B gives a sodium salt when treated with NaOH. Treatment of the sodium salt of B with...
-
When compound A is irradiated with ultraviolet light for 115 hours in pentane, an isomeric compound B is obtained that decolorizes bromine in CH2C12 and reacts with ozone to give, after the usual...
-
If isomer A is heated to about 100 °C, a mixture of isomers A and B is formed. Explain why there is no trace of isomer C or D. CDa CHs CD2 C6H5 CD3 Hs CD3 CH; 100 C CH3 CH CH C%H5 CHs
-
Phoenix Corp. faltered in the recent recession but is recovering. Free cash flow has grown rapidly. Forecasts made in 2016 are as follows. Phoenix's recovery will be complete by 2021, and there will...
-
Trotman Company had three intangible assets at the end of 2012 (end of the accounting year): (a) Computer software and Web development technology purchased on January 1, 2011, for $70,000. The...
-
Write an executive summary on the Maroochy Shire Sewage Spill case study. Your summary should not exceed one single spaced page and should include Who, What, When, Where, Why and How the situation...
-
Calculate overhead variances from the following data: Standard Actual Fixed overheads (Rs) 8,000 8,500 Variable overheads (Rs) 12,000 11,200 Output in units 4,000 3,800
-
A pipeline, used for the transport of crude oil, is buried in the earth such that its centerline is a distance of 1.5 m below the surface. The pipe has an outer diameter of 0.5 m and is insulated...
-
2 Rodney borrowed $15,000,000 for a modest vacation home in Lajolla, CA. The lender, Scrooge National Bank requires that he repay the loan in twenty installments of $ 1,103,726 The first payment is...
-
The reaction in Fig. P28.41 occurs as a sequence of two pericyclic reactions. Identify the intermediate A, and describe the two reactions. CH3 +BO_i_c_c_i_OF EtO-C- a-phellandrene Figure P28.41 OEt...
-
Classify the following sigmatropic reaction, give the curved- arrow notation, and show that the stereochemistry is that expected for a thermal concerted reaction. (This reaction, discovered by Prof....
-
Alisha Ali is in charge of maintenance. She is evaluated based on a flexible budget that uses the number of machine hours operated. Alisha gets a sizable bonus if her actual costs come in below...
-
See US Debt Clock and answer the following: (Hint: Take a screenshot of the Debt Clock) (2) A. What is the current US deficit and the total federal debt? (1) B What is the net interest...
-
GASB states that public colleges and universities are special purpose governmentsand therefore accountable to the citizenry (Hoyle, 2015). Furthermore, GASB found that for public colleges and...
-
You have recently been assigned to the production planning department within your company. Your firm makes large blades for power generation windmills. The windmills are mostly used in the western...
-
Jason Ready attended the University of Ohio from 2 0 1 9 to 2 0 2 3 under the Air Force ROTC program. Excluding the school expenses covered by his ROTC scholarship, he incurred additional school...
-
Questions Chap 1 1. Consider the following cases and decide whether criminal or civil proceedings would result, and make a note of the parties in the action. a) Ali is being prosecuted for careless...
-
My Life Chronicles Inc. collects 30% of its sales on account in the month of the sale and 70% in the month following the sale. If sales on account are budgeted to be $170,000 for June and $200,000...
-
A 2500-lbm car moving at 15 mi/h is accelerated at a constant rate of 15 ft/s 2 up to a speed of 50 mi/h. Calculate force and total time required?
-
Estimate each of the bond angles and order the bond lengths (smallest first) for each of the following molecule. (a) a) (b) d) Br:
-
(a) Give the H-C=O bond angle in methyl formate. (b) One dihedral angle in methyl formate relates the plane containing the O=C-O bonds to the plane containing the C-O-C bonds. Sketch two structures...
-
The compound benzenc has only one type of carbon-carbon bond, and this bond has a length intermediate between that of a single bond and a double bond. Draw a resonance structure of benzene that,...
-
Berbice Inc. has a new project, and you were recruitment to perform their sensitivity analysis based on the estimates of done by their engineering department (there are no taxes): Pessimistic Most...
-
#3) Seven years ago, Crane Corporation issued 20-year bonds that had a $1,000 face value, paid interest annually, and had a coupon rate of 8 percent. If the market rate of interest is 4.0 percent...
-
I have a portfolio of two stocks. The weights are 60% and 40% respectively, the volatilities are both 20%, while the correlation of returns is 100%. The volatility of my portfolio is A. 4% B. 14.4%...

Study smarter with the SolutionInn App


