When a deuterium-substituted cyclohexene is subjected to CyP450-promoted oxidation, two different allylic alcohols are formed. Explain. D
Question:
When a deuterium-substituted cyclohexene is subjected to CyP450-promoted oxidation, two different allylic alcohols are formed. Explain.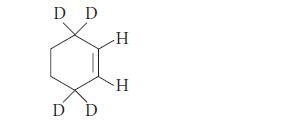
Transcribed Image Text:
D D D D H H
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
Remember that once the benzylic hydrogen or in this case deuterium ...View the full answer

Answered By

Nyron Beeput
I am an active educator and professional tutor with substantial experience in Biology and General Science. The past two years I have been tutoring online intensively with high school and college students. I have been teaching for four years and this experience has helped me to hone skills such as patience, dedication and flexibility. I work at the pace of my students and ensure that they understand.
My method of using real life examples that my students can relate to has helped them grasp concepts more readily. I also help students learn how to apply their knowledge and they appreciate that very much.
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
What is the effect of cultural commoditization and transformation on local traditions and customs? Give an example. Check Chapter 4 in the textbook for information on cultural commoditization and...
-
The Swern oxidation, shown in Fig. P10.61, is a very mild two-step procedure for the oxidation of primary and secondary alcohols. (a) How many electrons are involved in this oxidation? Explain. (b)...
-
Please help with the discusin questions ! I give thumbs up Case #1: Hailing a New Era: Haier in Japan As one of the most valuable brands in China, Haier designs,manufactures, and sells various home...
-
What would a profile look like across a restraining bend? Releasing bend?
-
Effects of gains and losses from sales of equipment on cash flows Exhibit 5.23 presents an abbreviated statement of cash flows for Largay Corporation for the current year (amounts in thousands)....
-
Abercrombie & Fitch sells casual apparel and personal care products for men, women, and children through retail stores located primarily in shopping malls. Its fiscal year ends January 31 of each...
-
Enter the following transactions in an Analytical Petty Cash Book under imprest system and balance it: 2003 Jan. 1 Received a cheque towards pretty cas ` 250 5 Paid taxi charge ` 60 8 Paid for...
-
MPA Worldwide Market Research found the average age in a random sample of adult moviegoers was 39 (source: commercialalert.org/moviemadem.htm). If the sample size was 1000 and the population standard...
-
Paul Swanson has an opportunity to acquire a franchise from The Yogurt Place, Incorporated, to dispense frozen yogurt products under The Yogurt Place name. Mr. Swanson has assembled the following...
-
(a) A compound A has the formula C 8 H 10 . After vigorous oxidation, it yields phthalic acid. What is the structure of A? (b) A compound B has the formula C 8 H 10 . After vigorous oxidation, it...
-
Explain how and why the product(s) would differ in the following reactions of trans-2-buten-1-ol. (1) Reaction with concentrated aqueous HBr (2) Conversion into the tosylate, then reaction with NaBr...
-
Jake borrowed $800,000 from the Gateway Bank to purchase a fishing boat. He keeps the boat at a dock owned by the Harbor Company. He uses the boat to earn income by fishing. Jake also has a contract...
-
Administrators at International University are curious how students' GPAs after their first year compare to their high school GPAs. They plan on taking an SRS of 80 of the 900 freshmen to look up...
-
( 8 x - x ^ 2 ) / ( x ^ 4 ) what is the derivate.
-
Solve for x . log 1 0 ( 4 x ) log 1 0 ( x 3 ) = 1
-
Let f ( x ) = ( 8 x - 4 x ^ 2 ) It is ^ x . Find the inflection points
-
f ( x ) = sin ( x ) / ( 2 * x ^ 2 + 4 ) , differentiate using quotient with respect to x
-
Explain why speeding up the collection of accounts receivable provides only a one-time increase in cash receipts.
-
Revol Industries manufactures plastic bottles for the food industry. On average, Revol pays $76 per ton for its plastics. Revol's waste-disposal company has increased its waste-disposal charge to $57...
-
From the molecular masses and the relative intensities of their M and M + 1 peaks, suggest molecular formulas for the following compounds. M (m/z = 82; 37%), M + 1 (2.5%); contains C and H.
-
Suggest a structure for each of the ions corresponding to the following peaks in the EI mass spectrum of ethyl bromide, and give a mechanism for the formation of each ion. (The numbers in parentheses...
-
The physical basis of some carbon monoxide detectors is the infrared detection of the unique stretching vibration of carbon monoxide at 2143 cm-1. How many times per second does this stretching...
-
You would like to have a balance of $600,000 at the end of 15 years from monthly savings of $900. If your returns are compounded monthly, what is the APR you need to meet your goal?
-
Explain the importance of covariance and correlation between assets and understanding the expected value, variance, and standard deviation of a random variable and of returns on a portfolio.
-
On August 1 , 2 0 2 3 , Mark Diamond began a tour company in the Northwest Territories called Millennium Arctic Tours. The following occurred during the first month of operations: Aug. 1 Purchased...

Study smarter with the SolutionInn App


