The Swern oxidation, shown in Fig. P10.61, is a very mild two-step procedure for the oxidation of
Question:
The Swern oxidation, shown in Fig. P10.61, is a very mild two-step procedure for the oxidation of primary and secondary alcohols.
(a) How many electrons are involved in this oxidation? Explain.
(b) What is the oxidizing agent?
(c) The following intermediate A is formed prior to the addition of the base triethylamine.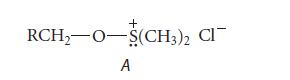
Triethylamine reacts with intermediate A to give the product aldehyde and the by-product dimethyl sulfide. When an isotopically substituted alcohol RCD2OH is subjected to the Swern oxidation, a deuteriumsubstituted aldehyde R—CD = O is formed, and the by-product dimethyl sulfide contains one atom of deuterium per molecule, H3C—S—CH2D. Write a curved-arrow mechanism for the reaction of intermediate A and the base triethylamine that accounts for the isotopic distribution in the products.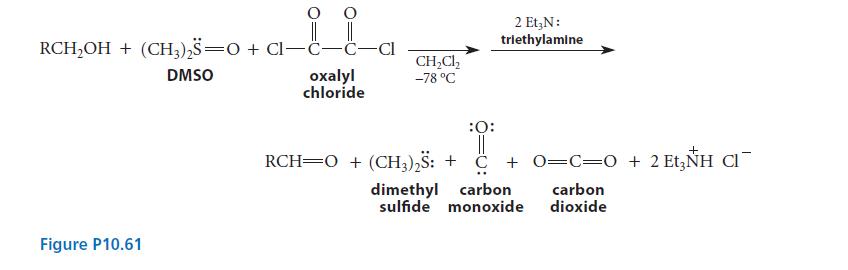
Step by Step Answer:






