Which alkyl halide and what conditions should be used to prepare the following alkene in good yield
Question:
Which alkyl halide and what conditions should be used to prepare the following alkene in good yield by an E2 elimination?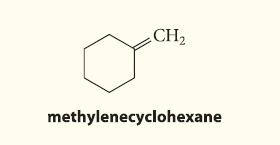
Transcribed Image Text:
CH₂ methylenecyclohexane
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (9 reviews)
If this alkene is to be produced in an E2 reaction from an alkyl halide the halide must be located a...View the full answer

Answered By

Muqadas Javed
I am a mentor by profession since seven years. I have been teaching on online forums and in universities. Teaching is my passion therefore i always try to find simple solution for complicated problems or task grasp them so that students can easily grasp them.I will provide you very detailed and self explanatory answers and that will help you to get good grade. I have two slogans: quality solution and on time delivery.
4.60+
24+ Reviews
144+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
What alkyl halide and what alkene would yield each of the following cyclopropane derivatives in the presence of a strong base? (a) Br Br (b) Ph H HC HC CH 3 CH3
-
Show how the Wittig reaction might be used to prepare the following alkenes. Identify the alkyl halide and the carbonyl components that would he used. (b) (a)
-
Financial statements for the ACTACTSTAT Company are given below: During 2021 the firm declared and paid cash dividends of P85,000. There were 50,000 shares of common stock outstanding throughout the...
-
GASB considers budgetary accounting and reporting to be important. List the principles outlined by GASB related to budgetary accounting and reporting.
-
TRM Consulting Services currently has the following capital structure: Source Book Value Quantity Common Stock........................ $ 8,500,000..................... 350,000 Preferred...
-
Distribute dividends between common stock and preferred stock. (p. 453) AppendixLO1
-
Tidy House produces a variety of household products. The firm operates 24 hours per day with three daily work shifts. The first-shift workers receive regular pay. The second shift receives an 8...
-
2. Tom's Tool Rentals began operations in 20x0. All income in the company classifies as 'business income'. The following information pertains to transactions in 20x0. --Tom signed an 8-year lease for...
-
What is the expected nucleophilic substitution product when (a) Methyl iodide reacts with Na + CH 3 CH 2 CH 2 CH 2 S ? (b) Ethyl iodide reacts with ammonia?
-
The crown ether [18]-crown-6 has a strong affinity for the methylammonium ion, CH 3 + NH 3 . Propose a structure for the complex between [18]-crown-6 and this ion. Show the important interactions...
-
Graph the equation by plotting points. x - y = 2
-
Find the best predicted tip for a ride that is 3.10 miles. How does the result compare to the actual tip of $4.55? Find the best predicted fare amount for a distance of 3.10 miles. How does the...
-
Since the SUTA rates changes are made at the end of each year, the available 2022 rates were used for FUTA and SUTA. Note: For this textbook edition the rate 0.6% was used for the net FUTA tax rate...
-
I have asked three questions with my textbook chapter in which they come from. it would be greatly appreciated if you could help me answer these three questions. Thanks so much. :) 1. What is the...
-
From your reading in Stevens & Smith (2010), a basic description of a medical detoxification, dual-diagnosis inpatient hospital, independent rehab programs, partial hospitalization, halfway houses,...
-
In Problems 1-4, indicate whether the given series converges or diverges. If it converges, find its sum. Hint: It may help you to write out the first few terms of the series? 1. 2. 3. 4. k-2 23(- k=1
-
Gordon and Lisa estimate that they will need $1,875,000 in 40 years for their retirement years. If they can earn 8 percent annually on their funds, how much do they need to save annually?
-
What product would expect to obtain from base treatment of 1,6-cyclo-decacedione? Base 1,6-Cyclodecanedione
-
Show the products you would except to obtain by claisen condensation of the following esters: (a) (CH3)2CHCH2COEt (b) Ethyl phenyl acetate (c) Ethyl cyclohexylacetate
-
As shown in figure 23.5, the Claisen reaction is reversible. That is, a ??keto ester can be cleared by base into two fragments. Using curved arrows to indicate electron flow, show the mechanism by...
-
A stock is expected to pay a dividend of $1.50 at the end of the year (i.e., D 1 = $1.50), and it should continue to grow at a constant rate of 10% a year. If its required return is 14%, what is the...
-
The Hobby Shop has a checking account with a ledger balance of $1,700. The firm has $2,400 in uncollected deposits and $4,200 in outstanding checks. What is the amount of the disbursement float on...
-
An investment will pay you $34,000 in 11 years. If the appropriate discount rate is 6.1 percent compounded daily, what is the present value? (Use 365 days a year. Do not round intermediate...

Study smarter with the SolutionInn App


