Which of the following alkenes can exist as double-bond stereoisomers? Identify the stereocenters in each. (a) H,C=CHCH,CH,CH,
Question:
Which of the following alkenes can exist as double-bond stereoisomers? Identify the stereocenters in each.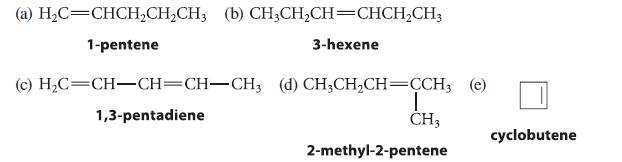
Transcribed Image Text:
(a) H,C=CHCH,CH,CH, (b) CH,CH,CH=CHCH,CH, 1-pentene 3-hexene (c) H₂C=CH-CH=CH-CH3 (d) CH3CH₂CH=CCH, (e) 1,3-pentadiene T CH3 2-methyl-2-pentene cyclobutene
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
a 1Pentene cannot exist as stereoisomers b 3Hexene can exist as both cis and trans iso...View the full answer

Answered By

Anthony Ngatia
I have three academic degrees i.e bachelors degree in Education(English & Literature),bachelors degree in business administration(entrepreneurship option),and masters degree in business administration(strategic management) in addition to a diploma in business management.I have spent much of my life in the academia where I have taught at high school,middle level colleges level and at university level.I have been an active academic essays writer since 2011 where I have worked with some of the most reputable essay companies based in Europe and in the US.I have over the years perfected my academic writing skills as a result of tackling numerous different assignments.I do not plagiarize and I maintain competitive quality in all the assignments that I handle.I am driven by strong work ethics and a firm conviction that I should "Do Unto others as I would Like them to do to me".
4.80+
76+ Reviews
152+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Which of the following alkenes can exist as double-bond stereo? Identify the stereo centers in each. (a) CH3CH2CH=====CHCH2CH3 (b) CH,CIH CH-CCH CH
-
Which of the following alkenes can exist as c is-trans isomers? Write their structures. Build hand-held models to prove that one isomer is not superposable on the other. (a) CH2==CHCH2CH3 (b)...
-
KYC's stock price can go up by 15 percent every year, or down by 10 percent. Both outcomes are equally likely. The risk free rate is 5 percent, and the current stock price of KYC is 100. (a) Price a...
-
The top 5 stocks in the S&P 500 index, when ranked by market capitalization, make up 22% of the total market capitalization of the S&P 500 index. Numerical estimates of the mean (or expected) rates...
-
Tata Consultancy of Bombay, India, is an international computer consulting firm. It spends considerable time and effort recruiting the best personnel from Indias leading technical schools. Tata...
-
Propylene, C3H6, is a gas that is used to form the important polymer called polypropylene. Its Lewis structure is (a) What is the total number of valence electrons in the propylene molecule? (b) How...
-
What is the difference between a shortsighted and a knowledgeable project sponsor? How can making this distinction help the project manager during project closure? AppendixLO1
-
A survey of 36 randomly selected iPhone owners showed that the purchase price has a mean of $416 with a sample standard deviation of $180. a. Compute the standard error of the sample mean. b. Compute...
-
USD = US Dollars BRL - Brazilian Reals January 2, 2020: USD-BRL = 4.2085 May 15, 2020: USD-BRL = 5.8071 INSTRUCTIONS ON HOW TO ENTER YOUR SOLUTIONS: 1) ROUND ALL FINAL ANSWERS IN USD OR BRL TO TWO...
-
Draw a conventional structure corresponding to the following skeletal structure, and then name it.
-
Name the following compound using IUPAC substitutive nomenclature. HC=CCH,CH,CH, I CH,CH,CH,CH,CH,
-
Consider the hydrogen-removal process described in Problem 14.46, but under conditions for which the mass diffusivity of the hydrogen gas (A) in the sheet material (B) is D AB = 1.8 x 10 -11 m 2 /s...
-
Complete Exercises 2-B and 2-H in Writing and Analysis in the Law using what you learned in the reading and in the Seminar. Use paragraph form, use complete sentences, and make sure you use proper...
-
What is the value of a stock expected to be in 9 years if the annual dividend is expected to remain unchanged forever at $3.65, the expected rate of return is 6.9% per year, and the next dividend is...
-
Once invested IN a corporation, shareholders want their money out - they want a return on investment! John owns 2 5 % of REFUND CORP INC, which paid out a $ 5 0 , 0 0 0 distribution to him on 1 2 / 3...
-
Worksheet Financial Statement Ratios. Lowe's Companies, Inc Jan 28, 2022 and Jan. 29, 2021 Current Ratio Current Assets / Current Liabilities Acid Test Current Assets Current Liabilities (Cash + ST...
-
3. Peter Senen operates in a JIT manufacturing system. For August, Peter Senen purchased 10,000 units of raw materials at P1.00 per unit on account.What is the The journal entry to record the...
-
Reports in the media about stem cells usually state that they turn into any kind of cell in the body. Explain why this statement is only partially correct, including a description of how a stem cell...
-
Havel says the grocer doesnt believe what is on the sign and indeed, he says the grocers customers will barely notice it. But Havel maintains that the sign serves a specific function. How would you...
-
Draw two constitutional isomers of cis-1, 2-dibromo-cyclopentane.
-
Draw a stereoisomer of trans-1, 3-climethylcyclobutane.
-
Hydrocortisone, a naturally occurring hormone produced in the adrenal glands, is often used to treat inflammation, severe allergies, and numerous other conditions. Is the indicated ?OH group in the...
-
Minden Company introduced a new product last year for which it is trying to find an optimal selling price. Marketing studies suggest that the company can increase sales by 5,000 units for each $2...
-
Prepare the adjusting journal entries and Post the adjusting journal entries to the T-accounts and adjust the trial balance. Dresser paid the interest due on the Bonds Payable on January 1. Dresser...
-
Venneman Company produces a product that requires 7 standard pounds per unit. The standard price is $11.50 per pound. If 3,900 units required 28,400 pounds, which were purchased at $10.92 per pound,...

Study smarter with the SolutionInn App


