Which of the following carbocations is more stable? + CH0-CH=CH-CH or A :CH, 1 HC=C-CH B +
Question:
Which of the following carbocations is more stable?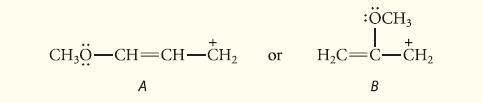
Transcribed Image Text:
+ CH₂0-CH=CH-CH₂ or A :ÖCH, 1 H₂C=C-CH₂ B +
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 85% (7 reviews)
The solution to this problem involves determining which carbocation has the greater number ...View the full answer

Answered By

PALASH JHANWAR
I am a Chartered Accountant with AIR 45 in CA - IPCC. I am a Merit Holder ( B.Com ). The following is my educational details.
PLEASE ACCESS MY RESUME FROM THE FOLLOWING LINK: https://drive.google.com/file/d/1hYR1uch-ff6MRC_cDB07K6VqY9kQ3SFL/view?usp=sharing
3.80+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
1. Summarize the law relied on by the judicial body in determining the issues of the case and arriving at its conclusion, by referencing the key statutory provision. like (e.g. name of Act,...
-
Each of the following carbocations has the potential to rearrange to a more stable one. Write the structure of the rearranged carbocation. (a) CH3CH2CH2+ (b) (c) (d) (CH3CH2)3CCH2+ (e) (CH32CHCHCHa...
-
Each of the following carbocations can rearrange to a more stable ion. Propose structures for the likely rearrangement products. H, (a) CH3CH2CH2CH2* (b) CH3CHCHCH3 CH CH CH2* (c)
-
One reason you might choose to sync a SharePoint library to your computer is to Select an answer: have a separate copy that your colleagues can't affect have a separate copy in case SharePoint breaks...
-
If cash is collected in advance of performing services, when is the associated revenue recognized?
-
Despite the economy, Liberty Bank has been investigating the possibility of initiating an internship program within our Financial Services Department. I have been appointed as the point person to...
-
LetX be an Ornstein-Uhlenbeck process with a long-run mean of zero; that is, dX = X dt + dB for constants and . Set Y = X2. Show that dY = ( Y)dt + Y dB for constants , and . Note: The squared...
-
Describe a situation in which the sales value at splitoff method cannot be used but the NRV method can be used for joint-cost allocation.
-
The inventory records for Tammy Company reflected the following Beginning inventory on May 1 1,200 units @ $4.00 First purchase on May 7 1,300 units @ $4.20 Second purchase on May 17 1,500 units 4.30...
-
The conjugated triene (E)-1,3,5-hexatriene has six molecular orbitals with relative energies 1.80, 1.25, and 0.44. (a) Sketch these MOs. Indicate which are bonding and which are antibonding. (b)...
-
Give the structure of the diene and dienophile that would react in a DielsAlder reaction to give the following product: COCH3 COCH3
-
Thirty samples of 16 cans each are measured from a canning process while it is in control. The mean of the 30 sample means is 12.03 ounces and the average range is 0.04 ounces. What should the upper...
-
Turn this information into an excel sheets with the excel formulas being shown P10.1 (LO 1) (Depreciation for Partial Period-SL, SYD, and DDB) Alladin Company purchased Machine #201 on May 1, 2025....
-
You are the Financial Analyst at Wellington Laboratories Ltd., a New Orleans, USA based bulk drugs manufacturer, which is evaluating the following project for manufacturing a new compound. Year Cash...
-
A variable mesh screen produces a linear and axisymmetric velocity profile as indicated below in the air flow through a 2-ft diameter circular cross section duct. The static pressures upstream and...
-
A vertical round steel rod 2 m long is securely held at its upper end. A weight can slide freely on the rod and its fall is arrested by a stop provided at the lower end of the rod. When the weight...
-
8) Determine the magnitudes of the forces F and P so that the single equivalent couple (i.e. the resultant of the three couples) acting on the triangular block is zero. Z -F F 3 m 10 N, 30 6 m 10 N 3...
-
Repeat (a) through (h) of problem 1 for the function f(x) = 1/x. (a) f(2) (b) f(2.1) (c) f(2.1) - f(2) (d) f(2.1) - f(2) / 2.1 - 2 (e) f(a + h) (f) f(a + h) - f(a) (g) f(a + h) - f(a)/(a + h) - a (h)...
-
Southwestern Punch was made by Frutayuda, Inc. and sold in 12-ounce cans to benefit victims of Hurricane Zero. The mean number of ounces placed in a can by an automatic fill pump is 11.7 with a...
-
Draw a segment of the polymer that might be prepared from 2-phenyl-1, 3-butadiene.
-
Show the mechanism of the acid-catalyzed polymerization of 1, 3-hutadiene.
-
Calculate the energy range of electromagnetic radiation in the UV region of the spectrum from 200 to 400 nm. How does this value compare with the values calculated previously for JR and NMR...
-
Slow Roll Drum Co. is evaluating the extension of credit to a new group of customers. Although these customers will provide $198,000 in additional credit sales, 13 percent are likely to be...
-
Wendell's Donut Shoppe is investigating the purchase of a new $39,600 conut-making machine. The new machine would permit the company to reduce the amount of part-time help needed, at a cost savings...
-
1.Discuss the challenges faced with Valuing Stocks and Bonds. As part of this discussion, how will the selected item be implemented in an organization and its significance? 2. Discuss how Valuing...

Study smarter with the SolutionInn App


