Give the structure of the diene and dienophile that would react in a DielsAlder reaction to give
Question:
Give the structure of the diene and dienophile that would react in a Diels–Alder reaction to give the following product: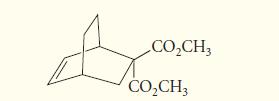
Transcribed Image Text:
CO₂CH3 CO₂CH3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 20% (5 reviews)
In the product of a DielsAlder reaction the two carbons of the double bond and the two adjacent carb...View the full answer

Answered By

Caroline Kinuthia
Taking care of the smaller details in life has a larger impact in our general well being, and that is what i believe in. My name is Carol. Writing is my passion. To me, doing a task is one thing, and delivering results from the task is another thing. I am a perfectionist who always take things seriously and deliver to the best of my knowledge.
4.90+
1934+ Reviews
4278+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Give the diene and dienophile that would react in a DielsAlder reaction to give each of the following products. (a) CN (b) COCH3
-
Give the diere and dienophile that would react in a Diels-Alderreaction to give each of the following products. CN
-
Waterways Corporation uses very stringent standard costs in evaluating its manufacturing efficiency. These standards are not "ideal" at this point, but the management is working toward that as a...
-
Select only 2. Two questions which can be used to address ethical issues impacting organizations and Human Resource Management include: What would my Supervisor say? Does this behavior or result meet...
-
What does the term deferral mean?
-
The following information is for the standard and actual costs for the Happy Corporation. Standard Costs: Budgeted units of production - 16,000 (80% of capacity) Standard labor hours per unit - 4...
-
Suppose dS/S = dt + dB for constants and and a Brownian motion B. Let r be a constant. Consider a wealth process W as defined in Section 12.2: dW W = (1 )r dt + dS S , where is a constant. (a)...
-
Repeat Problem 14 for the case when two of the positive charges, on opposite comers, are replaced by negative charges of the same magnitude (Fig. 16-51) -6.00 mC 0.100 m 6.00 mC 0.100 m 0.100 m 6.00...
-
what is income tax provision
-
Which of the following carbocations is more stable? + CH0-CH=CH-CH or A :CH, 1 HC=C-CH B +
-
An optically active alkyne A (C 10 H 14 ) can be catalytically hydrogenated to butylcyclohexane. Treatment of A with EtMgBr liberates no gas. Catalytic hydrogenation of A over Pd/C in the presence of...
-
Consider the description of Walkers Crisps' strategic marketing activity. How would you evaluate their actions? How would you compete against them?
-
PP Company purchases a material that is then processed to yield three chemicals: anarol, estyl, and betryl.In June, PPC purchased 10,000 gallons of the material at a cost of $250,000, and the company...
-
Suppose Boyson Inc. free cash flow for the next year is $ 1 5 0 , 0 0 0 and the FCF is expected to grow a concert rate of 6 . 5 % if WACC is 1 2 . 5 % what is the market value of the firm?
-
An eight lane urban freeway (four lanes in each direction) is on rolling terrain and has 11-ft lanes with a 4-ft right-side shoulder. The interchange density is 1.25 per mile. The base free-flow...
-
For the following business events, please indicate the increase (+) or decrease (-) on the following income statement and balance sheet categories. If there is no effect, leave the box blank. If...
-
4. Change the magnet to the original orientation and drag through the coil. a. What happens to the voltage and light bulb as the North Pole moves through the coil? b. What happens to the voltage and...
-
One airplane leaves an airport at noon flying north at 300 miles per hour. Another leaves the same airport one hour later and flies east at 400 miles per hour. (a) What are the positions of the...
-
What recommendations would you make to Big Four firms to help them (1) avoid confrontations with governmental officials in an authoritarian society and (2) deal effectively with such confrontations...
-
A knowledge of molar absorptivities is particularly important in biochemistry, where UV spectroscopy can provide an extremely sensitive method of analysis. For example, imagine that you wanted to...
-
Which of the following compounds would you expect to show ultraviolet absorptions in the 200 to 400nmrange? (a) (b) (c) CN CH3 (f) (d) (e) " N. Indole Aspirin
-
Show the structures of all possible adducts of the following diene with 1 equivalent ofHC1:
-
Which of the following programs covers custodial care? A HMOs B Medicare Part B C PPOs D Medicare Part A E Medicaid
-
uppose a taxpayer has exhausted his lifetime exclusion amount and has $14 million. a. Assuming a flat 40% gift tax rate, what is the maximum amount a taxpayer can transfer to her daughter (and still...
-
Physical Units Method, Relative Sales Value Method Farleigh Petroleum, Inc., is a small company that acquires high - grade crude oil from low - volume production wells owned by individuals and small...

Study smarter with the SolutionInn App


