Which of the following four structures represent constitutional isomers of the same molecule, and which one is
Question:
Which of the following four structures represent constitutional isomers of the same molecule, and which one is neither isomeric nor identical to the others? Explain your answers.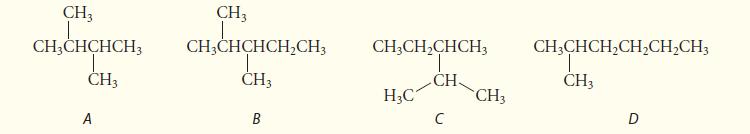
Transcribed Image Text:
CH3 CH3CHCHCH3 CH3 A CH3 CH3CHCHCH₂CH3 CH3 B CH3CH₂CHCH3 T CH H₂C C CH3 CH3CHCH₂CH₂CH₂CH3 CH3 D
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (4 reviews)
Compounds must have the same molecular formula to be either identical or isomeric Structure A has a ...View the full answer

Answered By

Raunak Agarwal
Teaching is my hobby and now my profession. I teach students of CA and CFA(USA) in batches of 100 students and have a 5 year experience.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
What did you learn about Essential Business Concepts?
-
Which of the following structures represent the same compound? Which ones represent different compounds? (a) (b) (c) (d) (e) (f) (g) Name the structures given in Problem 3-33, parts (a), (c), (e),...
-
An investor wishes to analyse the effects of different compounding frequencies Suppose 1000 is invested for 1 year at an interest rate of 5 per annum compounded Assume there are 365 days in 1 year
-
Suppose that Canada produces two goods: lumber and fish. It has 18 million workers, each of whom can cut 10 feet of lumber or catch 20 fish each day. a. What is the maximum amount of lumber Canada...
-
Researchers can draw various conclusions from a set of data. How do you know how to shape conclusions and recommendations?
-
A steel column of hollow circular cross section is supported on a circular steel base plate and a concrete pedestal (see figure). The column has outside diameter d = 250 mm and supports a load P =...
-
Web-based exercise. The Statistical Abstract of the United States is an essential compilation of data. You can find it online at the Census Bureau Web site. The most recent edition is at...
-
Calculating Annuity Values you are serving on a jury. A plaintiff is suing the city for injuries sustained after a freak street sweeper accident. In the trial, doctors testified that it will be five...
-
During December 2010. Carroll Company's employees earned $2,600 but the payroll will not cut until January 2020. Which of the following correctly portrays the adjusting entry needed in December...
-
(a) Draw a Newman projection for each staggered and eclipsed conformation about the C2C3 bond of isopentane, a compound containing a branched carbon chain. Show all staggered and eclipsed...
-
Suppose you take a trip to a distant universe and find that the periodic table there is derived from an arrangement of quantum numbers different from the one on Earth. The rules in that universe are...
-
In the Statement of Revenues, Expenditures, and Changes in Fund Balances, transfers must be reported a. in a separate section immediately following revenues. b. in a section immediately following the...
-
3. (30 pts total) Suppose that an automobile has the ability to accelerate from rest to a velocity of 100.0 mi/h in a time of 6.00 s. a. (15 pts) Assuming the acceleration is a constant, determine...
-
Factor completely. 2-2t+16
-
Use the following table to answer questions and ll]. The number of hot dogs sold by 12 randomly selected hot dogs vendors in Central Park on July 4 is as follows: 142 97 105 76 90 83 123 115 92 94 73...
-
Can you please describe (in about a paragraph) a situation (preferably but not necessarily in business) that requires a decision necessitating a decision tree with at least two branches, each branch...
-
Data: Sodium Systolic98 14799 14996 175109 14591 135107 14987 121110 170102 163103 141117 14992 13590 12793 132113 18199 152114 164103 14496 148111 180128 18392 13284 135102 141103 147117 16789...
-
Explain why cone vision is generally more acute than rod vision.
-
Describe a group you belong or have belonged discuss the stages of group development and suggest how to improve the group effectiveness by using the group development model.
-
Show all the steps in the mechanism for this reaction. What substitution product(s) would also be formed in thisreaction? Br E:OH
-
Show the products of this reaction. How would the composition of the products change if t-BuO ? in t-BuOH were used in place of ethoxide ion in ethanol? ELOH + CH,CH,0
-
All of the stereo isomers of 1, 2, 3, 4, 5, 6-hexachlorocyclohexane have very similar rates of E2 reaction except the following stereo isomer, which reacts about 7000 times more slowly than the...
-
Famas Llamas has a weighted average cost of capital of 8.8 percent. The companys cost of equity is 12 percent, and its pretax cost of debt is 6.8 percent. The tax rate is 22 percent. What is the...
-
The common stock of a company paid 1.32 in dividens last year. Dividens are expected to gros at an 8 percent annual rate for an indefinite number of years. A) If the company's current market price is...
-
(1 point) Bill makes annual deposits of $1900 to an an IRA earning 5% compounded annually for 14 years. At the end of the 14 years Bil retires. a) What was the value of his IRA at the end of 14...

Study smarter with the SolutionInn App


