Which of the following secondary alkyl halides reacts faster with CN in the S N 2
Question:
Which of the following secondary alkyl halides reacts faster with –CN in the SN2 reaction?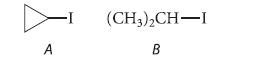
Transcribed Image Text:
A (CH3)2CH-I B
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
In the transition state of the S N 2 reaction the carbon at which substitution ...View the full answer

Answered By

Ashington Waweru
I am a lecturer, research writer and also a qualified financial analyst and accountant. I am qualified and articulate in many disciplines including English, Accounting, Finance, Quantitative spreadsheet analysis, Economics, and Statistics. I am an expert with sixteen years of experience in online industry-related work. I have a master's in business administration and a bachelor’s degree in education, accounting, and economics options.
I am a writer and proofreading expert with sixteen years of experience in online writing, proofreading, and text editing. I have vast knowledge and experience in writing techniques and styles such as APA, ASA, MLA, Chicago, Turabian, IEEE, and many others.
I am also an online blogger and research writer with sixteen years of writing and proofreading articles and reports. I have written many scripts and articles for blogs, and I also specialize in search engine
I have sixteen years of experience in Excel data entry, Excel data analysis, R-studio quantitative analysis, SPSS quantitative analysis, research writing, and proofreading articles and reports. I will deliver the highest quality online and offline Excel, R, SPSS, and other spreadsheet solutions within your operational deadlines. I have also compiled many original Excel quantitative and text spreadsheets which solve client’s problems in my research writing career.
I have extensive enterprise resource planning accounting, financial modeling, financial reporting, and company analysis: customer relationship management, enterprise resource planning, financial accounting projects, and corporate finance.
I am articulate in psychology, engineering, nursing, counseling, project management, accounting, finance, quantitative spreadsheet analysis, statistical and economic analysis, among many other industry fields and academic disciplines. I work to solve problems and provide accurate and credible solutions and research reports in all industries in the global economy.
I have taught and conducted masters and Ph.D. thesis research for specialists in Quantitative finance, Financial Accounting, Actuarial science, Macroeconomics, Microeconomics, Risk Management, Managerial Economics, Engineering Economics, Financial economics, Taxation and many other disciplines including water engineering, psychology, e-commerce, mechanical engineering, leadership and many others.
I have developed many courses on online websites like Teachable and Thinkific. I also developed an accounting reporting automation software project for Utafiti sacco located at ILRI Uthiru Kenya when I was working there in year 2001.
I am a mature, self-motivated worker who delivers high-quality, on-time reports which solve client’s problems accurately.
I have written many academic and professional industry research papers and tutored many clients from college to university undergraduate, master's and Ph.D. students, and corporate professionals. I anticipate your hiring me.
I know I will deliver the highest quality work you will find anywhere to award me your project work. Please note that I am looking for a long-term work relationship with you. I look forward to you delivering the best service to you.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Which of the following alkyl halides form a substitution product in an SN1 reaction that is different from the substitution product formed in an SN2 reaction? a. b. c. d. e. f. CH Br CH CHCHCHCH CHa...
-
Which of the following two alkyl halides would react most rapidly in a solvolysis reaction by the SN1 mechanism? Explain your reasoning. Ch 1,0-CH-CH-CH,-Cl A (trans isomer) CH.O-C-CH,-CI CH2
-
When Brianna meets with her planner, he notes that her individual financial plan can be organized using four basic tax - planning strategies. These would include deferral, reduction, splitting, and:...
-
Solve: [1+ log (xy)] dx + [ 1 + x/y ]dy = 0.
-
Using the information provided in exercise 64, prepare the reconciliation of operating income to net cash provided by operating activities that would appear at the bottom of the December 31 Statement...
-
Below you have been provided the prices for Citigroup and the S&P 500 Index. a. Calculate the monthly holding-period returns for Citigroup and the S&P 500 Index. b. What are the average monthly...
-
On June 1, 2016, Sid was admitted to the partnership when he purchased, for $132,000, a proportionate interest from New and Sha in the net assets and profits of the partnership. As a result of this...
-
It has been reported that women end up unhappier than men later in life, even though they start out happier (Yahoo News, August 1, 2008). Early in life, women are more likely to fulfill their family...
-
s 12 Pointsl Under what conditions forward exchange rate is an unbiased estimator of Future spot rates. (Show all calculations to support your answer)
-
Tert-butyl chloride undergoes solvolysis in either acetic acid or formic acid. Both solvents are protic, donor solvents, but they differ substantially in their dielectric constants . (a) What is the...
-
What products are expected, including their stereochemistry, when (2S,3R)-2-bromo-3-methylpentane is subjected to each of the following conditions? Explain. (a) Methanol containing an excess of...
-
Why are raw strings often used when creating Regex objects?
-
Zephyr Minerals completed the following transactions involving machinery. Machine No. 1550 was purchased for cash on April 1, 2020, at an installed cost of $75,000. Its useful life was estimated to...
-
Kelly is a self-employed tax attorney whose practice primarily involves tax planning. During the year, she attended a three-day seminar regarding new changes to the tax law. She incurred the...
-
At a recently concluded Annual General Meeting (AGM) of a company, one of the shareholders remarked; historical financial statements are essential in corporate reporting, particularly for compliance...
-
4. In hypothesis, Mr. Ng wants to compare the solution in Q3 to other solutions in different conditions. If the following constraints are newly set in place, answer how much different is going to be...
-
3C2H6O2+7H2O= C2H4O3+11H2+O2+H2C2O4+CH2O2 Glycolic acid is produced electrochemically from ethylene glycol under alkaline conditions(NaOH). Hydrogen is produced at the cathode, and formic acid and...
-
Suppose that limn( nan = 1. Prove that (an diverges?
-
The National Collegiate Athletic Association (NCAA) and the National Federation of State High School Associations (NFHS) set a new standard for non-wood baseball bats. Their goal was to ensure that...
-
Assign R or S configuration to each chirality center in the following biologicalmolecules: (a) (b) .C 'N' N-N - CH2CH2CH2CH2CO2 Prostaglandin E, Biotin
-
Draw tetrahedral representations of the following molecules: (a) (S)-2-Chlorohutane (b) (R)-3-Chloro-1-pentene
-
Draw tetrahedral representations of the two enantiomers of the amino acid cysteine, HSCH 2 CH (NH 2 ) CO 2 H, and identify each as R or S.
-
When credit terms for a sale are 2/15, n/40, the customer saves by paying early. What percent (rounded) would this savings amount to on an annual basis
-
An industrial robot that is depreciated by the MACRS method has B = $60,000 and a 5-year depreciable life. If the depreciation charge in year 3 is $8,640, the salvage value that was used in the...
-
What determines a firm's beta? Should firm management make changes to its beta? Be sure to consider the implications for the firm's investors using CAPM.

Study smarter with the SolutionInn App


