Which of the following species should be aromatic by the Hckel 4n + 2 rule? (a) thiophene
Question:
Which of the following species should be aromatic by the Hückel 4n + 2 rule?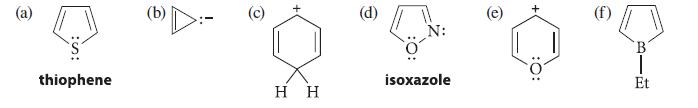
Transcribed Image Text:
(a) thiophene G O H H (d) N: isoxazole :0: B Et
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
a b d Thiophene is aromatic One electron pair on sulfur is part of the aromatic 7electron system the ...View the full answer

Answered By

Evans Cherono
I am an Information Technology Graduate and willing to work on any computer science or IT work to ensure I do my best all the time.
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Which of the compounds or ions in Problem 15.38 are likely to be antiaromatic? Explain. Problem 15.38 Which of the following species should be aromatic by the Hckel 4n + 2 rule? (a) thiophene G O H H...
-
Which d the following species should be aromatic by the Huckel 4n + 2 rule? (a) (b) (c) N: isoxazole C2Hs
-
Which of the following species (there may be more than one) is are likely to have the structure shown here: (a) XeF4 (b) BrFe4+ (c) SiF4 (d) TeCI4 (e) HClO4? (The colors do not reflect atom...
-
Problem 5: Closing entries. (10 points) Given the following accounts, prepare the closing entries in the order and method taught using Income Summary. Number each entry 1, 2, .... Accounts payable $...
-
Give an example of a cost that can be directly matched with the revenue produced by an accounting firm from preparing a tax return.
-
1. According to Porter's framework, what generic strategy was Airborne Express pursuing? Was this a sound strategy in the context of the air express industry? 2. What were the strengths of Airborne...
-
Quantum tunneling. At temperatures approaching absolute zero 1 -273C2, helium exhibits traits that seem to defy many laws of Newtonian physics. An experiment has been conducted with helium in solid...
-
The accounting (not the income tax) records of Consolidated Publications, Inc., provide the comparative income statement for 2010 and 2011, respectively: Taxable income for 2010 includes these...
-
Ca-Consumption after tax Sa - Savings after tax Remember: Tax is a constant value of $25
-
Using the theory of aromaticity, explain the finding that A and B are different compounds, but C and D are identical. (That A and B are different molecules was established by Prof. Barry Carpenter...
-
How many bonding MOs are there in a planar, cyclic, conjugated hydrocarbon that contains a ring of 10 carbon atoms? How many electrons does it have? How many of the electrons can be accommodated in...
-
Solve the following equations. 0.5( x 3) = 20
-
Companies that invest heavily in eco-friendly initiatives, such as transitioning to renewable energy sources or implementing carbon offset programs, may initially face increased operational costs....
-
Answer each question individually please. 14-13 What are the advantages and drawbacks of universities using social media to communicate with various stakeholdersstudents, potential students, alumni,...
-
act as a consultant hired by the operations director of the Barry Computer Company provide a financial analysis and comparison to the industry. You will conduct a financial ratio analysis to gain a...
-
Building a sense of community is not just a moral thing to do, but also a pragmatic one. In today's competitive and ever-evolving business environment, the organizations that can attract the most...
-
Watch https://youtu.be/U3MtvvNjUR4 What do you think of Dr. Saint's ideas about barriers to change? What do you think about social learning? Could this tool be used to make real change? How can the...
-
Find all points on the graph of y = x + sin x where the tangent line us parallel to the line y = 2 + x.
-
Two mutually exclusive investment alternatives are being considered. Alternative A requires an initial investment of $20,000 in a machine. Annual operating and maintenance costs are anticipated to be...
-
Carvone is an unsaturated ketone responsible for the odor of spearmint. If carvone has M = 150 in its mass spectrum and contains three double bonds and one ring, what is its molecular formula?
-
Carvone (Problem 12.39) has an intense infrared absorption at 1690 cm1. What kind of ketone does carvone contain?
-
The (a) mass spectrum and the (b) infrared spectrum of an unknown hydrocarbon are shown. Propose as many structures as youcan. (a) 100 80 60 40 20 10 20 40 60 80 100 120 140 m/z (b) 60 40 20 - 4000...
-
Suppose I have computed the cost of carbon per mile for my car at 0 . 0 1 2 per mile. Assume that the interest rate is 4 % and that I drive the car 2 8 , 0 0 0 miles per year. What is the present...
-
Imagine that in stable growth period, the firm earns ROIC of 10% and has after tax EBIT of 200 and reinvestment $ of 40. What is the steady state growth rate? 20% O 10% 2%
-
Tanner-UNF Corporation acquired as a long-term investment $160 million of 5.0% bonds, dated July 1, on July 1, 2021. Company management has the positive intent and ability to hold the bonds until...

Study smarter with the SolutionInn App


