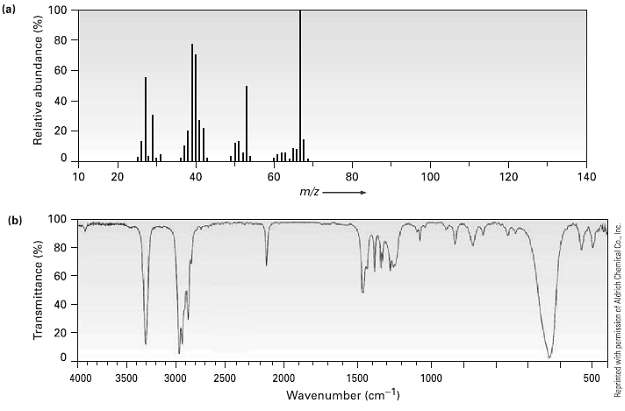
The (a) mass spectrum and the (b) infrared spectrum of an unknown hydrocarbon are shown. Propose as
Question:
The (a) mass spectrum and the (b) infrared spectrum of an unknown hydrocarbon are shown. Propose as many structures as youcan.
Transcribed Image Text:
(a) 100 80 60 40 20 10 20 40 60 80 100 120 140 m/z (b) 60 40 20 - 4000 3500 3000 2500 2000 1500 1000 500 Wavenumber (cm-1) Transmittance (%) Relative abundance (%) Reprinted with permissicn et Aldrich Chemical Co. Inc.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (8 reviews)
The peak of maximum intensity base peak in the mass spectrum occurs at mz 67 This ...View the full answer

Answered By

Seema kuldeep
although I don't have an experience of teaching in a particular institute, previously I was an expert on Chegg and I have used to teach my batch mates and also my juniors.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
The mass spectrum and infrared spectrum of an unknown compound are shown in Figures 13.27 and 13.28, respectively. Identify the compound. Figure 13.27 The mass spectrum for Problem 28. 100 E 80 3 60...
-
An unknown compound gives a mass spectrum with a weak molecular ion at m/z 113 and a prominent ion at m z 68. Its NMR and IR spectra are shown here. Determine the structure, and show how it is...
-
An unknown, foul-smelling hydrocarbon gives the mass spectrum and infrared spectrum shown. (a) Use the mass spectrum to propose a molecular formula. How many elements of unsaturation are there? (b)...
-
Reply as to whether you believe the following statements are correct (C) or incorrect (I) concerning PPS sampling. a. The size of a PPS sample is not based on the estimated variation of audited...
-
An article on price wars by two McKinsey consultants makes the following argument. That the (tit-for-tat) strategy is fraught with risk cannot be overemphasized. Your competitor may take an...
-
Galaxies tend to be strong emitters of Lyman- photons (from the n = 2 to n = 1 transition in atomic hydrogen). But the intergalactic medium the very thin gas between the galaxies tends to absorb...
-
Using the data from Problem 11.16, determine which means are different from one another using a = 0.05. AppendixLO1
-
Foxx Companys cost structure is dominated by variable costs with a contribution margin ratio of .25 and fixed costs of $100,000. Every dollar of sales contributes 25 cents toward fixed costs and...
-
Just 5~11 HelpPlease be more specific. Just 5~11. Thank you! Benjamin Moore is a national paint manufacturer and retailer. The company is segmented into five divisions: Paint Stores (branded retail...
-
Find the indicated probabilities using the geometric distribution, the Poisson distribution, or the binomial distribution Then determine if the events are unusual. If convenient, use the appropriate...
-
Carvone (Problem 12.39) has an intense infrared absorption at 1690 cm1. What kind of ketone does carvone contain?
-
The (a) mass spectrum arid the (b) infrared spectrum of another unknown hydrocarbon is shown. Propose as many structures as youcan. (a) 100 80 60 40 20 20 60 80 100 120 140 10 40 m/z 100 (b) * 80 60...
-
If you are in charge of a private firm and it doesnt have a share price, what should be your goal as a financial manager? Explain.
-
What is the discount rate? PV = 7 0 0 ; t = 5 year period; FV = 1 0 0 0
-
How is planning illustrated in this case story? How is strategic management illustrated in this case story? The new CEO stated that the CEO's job is to give employees a point of view. Explain what...
-
Explain the Following Questions: 1. What essential characteristics exist in a proper understanding of "personal mastery," so that as an individual achieves greater progress in this discipline, they...
-
Few people want to eat discolored french fries. Potatoes are kept refrigerated before being cut for french fries to prevent spoiling and preserve flavor. But immediate processing of cold potatoes...
-
Part 3 of 4 Points: 0.49 of 1 Compute P(X) using the binomial probability formula. Then determine whether the normal distribution can be used to estimate this probability. If so, approximate P(X)...
-
Should other variables be examined in the employee and customer projects? If yes, what specifically are these variables?
-
Refrigerant R-12 at 30C, 0.75 MPa enters a steady flow device and exits at 30C, 100 kPa. Assume the process is isothermal and reversible. Find the change in availability of the refrigerant.
-
The Safe Drinking Water Act (SDWA) sets a limit for mercurya toxin to the central nervous systemat 0.0020 ppm by mass. Water suppliers must periodically test their water to ensure that mercury levels...
-
Dimethylamine, (CH3)2NH has a molecular weight of 45 and a boiling point of 7.4 C. Trimethylamine, (CH3)3N has a higher molecular weight (59) but a lower boiling point (3.5 C). Explain this apparent...
-
Predict which member of each pair will be more acidic. Explain your answers. (a) Methanol or tert-butyl alcohol (b) 2-chloropropan-1-ol or 3-chloropropan-1-ol (c) 2-chloroethanol or...
-
Without looking them up, rank the following compounds in decreasing order of acidity. These examples represent large classes of compounds that differ widely in acidity. water, ethanol,...
-
[ The following information applies to the questions displayed below ] Nauticat has two classes of stock authorized: $ 1 0 par preferred, and $ 1 par value common. As of the beginning of 2 0 2 1 , 1...
-
Selling is not the most important part of marketing. Explain why not
-
When direct materials are issued from the storeroom, are any entries made in the subsidiary records? Question 2 options: Increase raw material item record Decrease raw material item record No entry...

Study smarter with the SolutionInn App


