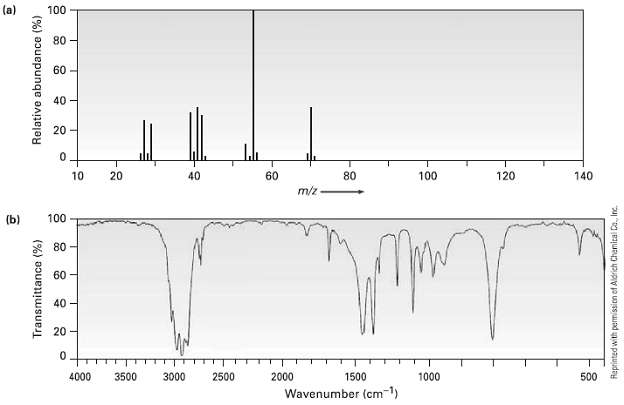
The (a) mass spectrum arid the (b) infrared spectrum of another unknown hydrocarbon is shown. Propose as
Question:
The (a) mass spectrum arid the (b) infrared spectrum of another unknown hydrocarbon is shown. Propose as many structures as youcan.
Transcribed Image Text:
(a) 100 80 60 40 20 20 60 80 100 120 140 10 40 m/z 100 (b) * 80 60 40 20 4000 3500 3000 2500 2000 1500 1000 500 Wavenumber (cm1) Transmittance (%) Relative abundance (%) Raprinted with permission ef Aldrich Chanical Co., Inc.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 53% (13 reviews)
The molecular ion M 70 corresponds to the molecular formula C5H10 This compou...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
The mass spectrum and infrared spectrum of an unknown compound are shown in Figures 13.27 and 13.28, respectively. Identify the compound. Figure 13.27 The mass spectrum for Problem 28. 100 E 80 3 60...
-
The mass spectrum of unknown compound A shows a molecular ion at m/z 116 and prominent peaks at m/z 87 and m/z 101. Its UV spectrum shows no maximum above 200 nm. The IR and NMR spectra of A follow....
-
Propose reasonable fragmentation mechanisms that explain why The EI mass spectrum of benzoic acid shows major peaks at m/z = 105 and m/z = 77.
-
An amplifier has three cascaded stages of amplification, each having available power gain of 10 dB and noise figure of 3 dB. i) ii) Calculate the noise factor (ratio), and noise figure (dB). If the...
-
Why are the Cournot and Bertrand models considered static? What aspects of real world behavior might be missing in static models?
-
FIGURE Q21.4 shows the pV diagram of a heat engine. During which stage or stages is (a) Heat added to the gas, (b) Heat removed from the gas, (c) Work done on the gas, (d) Work done by the gas? P2-...
-
Consider the following data collected for a randomized block ANOVA: Block Sample 1 Sample 2 Sample 3 1 8 4 3 2 5 4 3 3 6 2 1 4 13 9 5 a. Calculate the total sum of squares (SST). b. Partition the...
-
ExerWise, a new company marketing a high-end ab toner exercise machine, is considering direct marketing versus selling through Strongs, a national sporting goods retailer. As the buyer for Strongs,...
-
derivation of black model 7 6 for put for future contract
-
Assume that Valhalla decides to make the investment: What valuation do you think is appropriate at an assumed discount rate of 50%? What would be Valhallas expected IRR from the investment at a $5...
-
The (a) mass spectrum and the (b) infrared spectrum of an unknown hydrocarbon are shown. Propose as many structures as youcan. (a) 100 80 60 40 20 10 20 40 60 80 100 120 140 m/z (b) 60 40 20 - 4000...
-
Propose structures for compounds that meet the following descriptions: (a) An optically active compound C5H10O with an IR absorption at 1730 cm1 (b) A nonoptically active compound C5H9N with an 1k...
-
Sketch the root loci for the control system shown in Fig. P5.5(a). Determine the range of parameter \(\mathrm{K}\) for stability. Apply the angle criterion to show that root locus branches consist of...
-
Encouraging you to sit back and watch a full hour of one of your favorite shows on prime-time television. However, instead of getting up during the commercial break or fast forwarding through the...
-
A family member has been recently diagnosed with a heart condition that requires replacing a heart valve. She points out that if she goes to India, the surgery cost is about 60% cheaper on average...
-
Based on the case of Bowers Machine Parts. Critically analyze why people were not doing their best and critically explain why hiring a consultant might solve the issue. Justify your answer by using...
-
Identify a few strategies for sustainability effectiveness. Should sustainability be a corporation's top priority? Why or why not? What are the challenges associated with implementing sustainable...
-
Answer the following questions for the topic you want to write about. Type your answers in a separate Word document. What is the issue or debatable idea you might write about? What is debatable about...
-
What kind of research design would be most appropriate for the three projects, exploratory, descriptive, causal, or more than one type of research design? Justify your choice.
-
1-Stern observed all of the following results EXCEPT _______ in his experiment. A-one of the recombinant phenotypes was associated with an X chromosome of normal length B-the number of car, B+ male...
-
A gas has a Henrys law constant of 0.112 M/atm. What total volume of solution is needed to completely dissolve 1.65 L of the gas at a pressure of 725 torr and a temperature of 25 C?
-
A nitro group( - NO2) effectively stabilizes a negative charge on an adjacent carbon atom through resonance: Two of the following nitrophenols are much more acidic than phenol itself. The third...
-
Classify each reaction as an oxidation, a reduction, or nether. (a) CH3 - CH2OH (b) (c) (d) (e) (f) g) (h) (i) (j) (k) (l) CrOs pyridine CH H2CrO4 CH3CH -- CH3 H3C CH3 CH3---CH3 LiAIH TiCI...
-
Show how you would convert propan-1-ol to the following compounds using tosylate intermediate. You may use whatever additional reagents are needed. (a) 1-bromopropane (b) Propan-1-1amine,...
-
DETAILS 1. [-/1 Points) SMITHNM13 11.2.025. MY NOTES Convert the credit card rate to the APR. Oregon, 2% per month % Need Help? ReadIt Watch
-
Corom Stack Standard CALCULATOR PRINTER VERSION BACK NEXT Problem 13-02A a-c (Part Level Submission) Sheffield Corporation had the following stockholders' equity accounts on January 1, 2020: Common...
-
Suppose that you own 2,100 shares of Nocash Corp. and the company is about to pay a 25% stock dividend. The stock currently sells at $115 per share. a. What will be the number of shares that you hold...

Study smarter with the SolutionInn App


