Which of the following two carboxylic acids is more acidic? Explain. HC=CH-CH- -OH 3-butenoic acid H3C-CH-CH- butanoic
Question:
Which of the following two carboxylic acids is more acidic? Explain.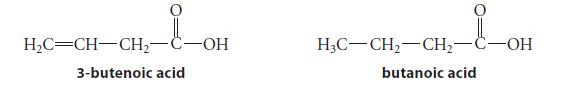
Transcribed Image Text:
H₂C=CH-CH₂- -OH 3-butenoic acid H3C-CH₂-CH₂- butanoic acid -OH
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (4 reviews)
As stated in the text above the problem the polar e...View the full answer

Answered By

PRINCE PANDEY
I am Indian Chartered Accounting having a strong hold in the subjects of Accounting, IFRS Reporting, Indian
Taxation, Cost Accounting, Auditing. I have vast experience of teaching a student with easy way problem-solving approach.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Chemists know that nitric and sulfuric acids are strong acids and that acetic acid is a weak acid. They would also agree that ethanol is at best a very weak acid. Acid strength is given directly by...
-
Proteins are synthesized with a particular amino acid sequence through the translation of information encoded in messenger RNA by an RNAprotein complex called a ribosome. Amino acids are specified by...
-
The following data were taken from the records of Menendez Company: Current assets Property, plant, and equipment Current liabilities Long-term liabilities Owner's equity $5,400 10,500 3,400 4,800...
-
The registers values in AX and BX can be exchanged using stack operations that are: Select one: a. PUSH bx PUSH ax POP bx POP AX b. none C. PUSH ax PUSH bx POP ax POP bx d. PUSH ax POP ax PUSH bx POP...
-
Boeken testified that he read the Surgeon Generals warning about smoking in the 1960s, so how can he argue fraud?
-
Ortho-dichlorobenzene, C6H4Cl2, is obtained when two of the adjacent hydrogen atoms in benzene are replaced with Cl atoms. A skeleton of the molecule is shown here. (a) Complete a Lewis structure for...
-
Describe the three approaches to releasing a product or implementing a system. AppendixLO1
-
1. You are in charge of strategic planning for Grand Casinos. The company wants to open and manage a casino in rural Iowa. Community residents have asked you and your strategic planning team to...
-
You are required to provide a report based on analysis sourced from recent text books and academic Briefly explain the following terms with the double-entry effects. Accrual Expenses (2marks) Prepaid...
-
Which compound in each set should have the larger dipole moment? Explain. (a) Cis-2-butene or trans-2-butene (b) Propene or 2-methylpropene
-
A compound has the molecular formula C 2 0 H 34 O 2 . Certain structural evidence suggests that the compound contains two methyl groups and no carboncarbon double bonds. Give one structure consistent...
-
New Wave Images is a graphics design firm that prepares its financial statements using a calendar year. Manny Kinn, the company treasurer and vice president of finance, has prepared a classified...
-
Account is a domestic growth portfolio. The current holdings are primarily US exchange-traded stocks and bonds. To remain in compliance, the total portfolio may only invest up to a maximum of 5% in...
-
The President of the United States needs your help. He has asked you to investigate and find answers to several important questions. His questions are included in the Letter from the President below....
-
Cheng Co. reports the following information for the coming year. Labor rate, including fringe benefits Annual labor hours Annual materials purchases Annual overhead costs: Materials purchasing,...
-
Compared to most objects, sound waves travel very fast. It is fast enough that measuring the speed of sound is a technical challenge. One method you could use would be to time an echo. For example,...
-
Sharif and Judith are married and purchased a vacation home together in Maine for $ 2 5 0 , 0 0 0 . Sharif died suddenly six months later and at that time the fair market value of the vacation home...
-
Describe the function of a ribosome in protein synthesis.
-
Suppose the S&P 500 futures price is 1000, = 30%, r = 5%, = 5%, T = 1, and n = 3. a. What are the prices of European calls and puts for K = $1000? Why do you find the prices to be equal? b. What...
-
Construct a qualitative potential-energy diagram for rotation about the CC bond of 1, 2-dibromoethane. Which conformation would you expect to be more stable? Label the anti and gauche conformations...
-
Which conformation of 1, 2-dibrornoethane (Problem 3.44) would you expect to have the larger dipole moment? The observed dipole moment of 1, 2-dibromoethane is = 1.0 D. What does this tell you about...
-
The barrier to rotation about the CC bond in bromoethane is 15kJ/mol (3.6kcal/mol). (a) What energy value can you assign to an HBr eclipsing interaction? (b) Construct a quantitative diagram of...
-
Flexible manufacturing places new demands on the management accounting information system and how performance is evaluated. In response, a company should a. institute practices that reduce switching...
-
Revenue and expense items and components of other comprehensive income can be reported in the statement of shareholders' equity using: U.S. GAAP. IFRS. Both U.S. GAAP and IFRS. Neither U.S. GAAP nor...
-
Kirk and Spock formed the Enterprise Company in 2010 as equal owners. Kirk contributed land held an investment ($50,000 basis; $100,000 FMV), and Spock contributed $100,000 cash. The land was used in...

Study smarter with the SolutionInn App


