Which of the non-hydrogen atoms in each of the following species has a complete octet? What is
Question:
Which of the non-hydrogen atoms in each of the following species has a complete octet? What is the formal charge on each? Assume all unshared valence electrons are shown.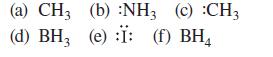
Transcribed Image Text:
(a) CH3 (b) :NH3 (c) :CH3 (d) BH, (e) :Ï: (f) BH4 3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)
The formal charge on all the hydrogens is 0 For the other atoms a Carbon ...View the full answer

Answered By

Susan Juma
I'm available and reachable 24/7. I have high experience in helping students with their assignments, proposals, and dissertations. Most importantly, I'm a professional accountant and I can handle all kinds of accounting and finance problems.
4.40+
15+ Reviews
45+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Which of the atoms in each of the following species has a complete octet? What is the formal charge on each? Assume all unshared valence electrons are shown. (a) CH (b) :NH3 (c) :CH3 (d) BH3 (e) :T:...
-
Below are the equations for four impulse responses. The plot below shows four step responses. Using the Laplace convolution technique, which impulse response goes with which step response (A, B, C,...
-
Give the formal charge on each atom and the net charge on each species in the following structures. All unshared valence electrons are shown. (a) (b) (c) CH3 trimethylamine oxide methylene :Cl-o:...
-
Sunblessed Juice Company sells bags of oranges and cartons of orange juice. Sunblessed grades oranges on a scale of 1 (poor) to 10 (excellent). At present, Sunblessed has 100,000 pounds of grade 9...
-
Drawing a Line Chart Your Task. Prepare a line chart showing the sales of Sidekick Athletic Shoes, Inc., for these years: 2008, $6.7 million; 2007, $5.4 million; 2006, $3.2 million; 2005, $2.1...
-
A bar ABC of length L consists of two parts of equal lengths but different diameters. Segment AB has diameter d1 = 100 mm, and segment BC has diameter d2 = 60 mm. Both segments have length L/2 = 0.6...
-
Explain the use of marketing ROI, metrics, and dashboards in evaluating marketing programs.
-
Parker Products Inc, a manufacturer, reported $ 123 million in sales and a loss of $ 18 million in its annual report to shareholders. According to a CVP analysis prepared for management, the companys...
-
Qu 10 QUESTION 18 The following information is available from the accounting records of the Bright Company Beginning balance of fixed assets $ 100.000 Net sales revenue 360,000 Ending balance of...
-
Draw one Lewis structure for each of the following compounds; show all unshared electron pairs. None of the atoms in the compounds bears a formal charge, and all atoms have octets (hydrogens have...
-
(a) Construct a hybrid orbital picture for the water molecule using oxygen sp 3 hybrid orbitals. (b) Predict any departures from tetrahedral geometry that you might expect from the presence of two...
-
Choosing among renting, leasing, and purchasing an AIS is strictly a financial decision and should be done by the finance staff. Do you agree? Discuss fully.
-
Solve for "C" and "E": 1) E cos (15)-C=0 2) -300+ C+E sin (15) = 0
-
Let u=3, b. Compute uv, uv, 2-3 v =
-
Using Complex Numbers show that d cosz=-sinz dz
-
use for loops to solve the following problems 1. Write a complete C++ program that does the following. It asks the user to enter their age (which is assumed to be a positive integer). The program...
-
Profile Vickers hardness test Penetrating body: Square diamond pyramid :Test force F N ... 981 N (HV 5 ... HV 100) 49 :Measured value Diagonals of the square impression d Hardness value: F 0,189 F...
-
Match each sensory receptor to the type of stimulus to which it is likely to respond: (1) Chemoreceptor .................. A. approaching headlights (2) Pain receptor .................... B. a change...
-
What are the risks and liability factors in an audit? What are the implications to the auditor? What are the implications to the organization? How can the auditor mitigate these risks and liability...
-
Show how these compounds could be synthesized from alkylhalides: a) Heptane b) PHCH,CH,CH,NH, c) PHCH,C=CH d) e) HO, OH f) g) H.C
-
Show how this synthesis might beaccomplished: Br CH3 CH3 HO from "CN -
-
What is wrong with these reactions explain. CI + NaOCH3 OCH3 + NaCl a) + HBr Br . b) CH,OH + CH;0 OCH3 CI d)
-
Suppose I have computed the cost of carbon per mile for my car at 0 . 0 1 2 per mile. Assume that the interest rate is 4 % and that I drive the car 2 8 , 0 0 0 miles per year. What is the present...
-
Imagine that in stable growth period, the firm earns ROIC of 10% and has after tax EBIT of 200 and reinvestment $ of 40. What is the steady state growth rate? 20% O 10% 2%
-
Tanner-UNF Corporation acquired as a long-term investment $160 million of 5.0% bonds, dated July 1, on July 1, 2021. Company management has the positive intent and ability to hold the bonds until...

Study smarter with the SolutionInn App


