Write a Brnsted acidbase reaction in which act as conjugate acidbase pairs. H/-:H and CH,H/CH,:-
Question:
Write a Brønsted acid–base reaction in which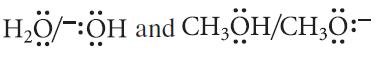
act as conjugate acid–base pairs.
Transcribed Image Text:
H₂Ö/-:ÖH and CH,ÖH/CH,Ö:-
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (5 reviews)
Let H 2 O be the acid on the left Then CH 3 OH ...View the full answer

Answered By

Keziah Thiga
I am a self motivated financial professional knowledgeable in; preparation of financial reports, reconciling and managing accounts, maintaining cash flows, budgets, among other financial reports. I possess strong analytical skills with high attention to detail and accuracy. I am able to act quickly and effectively when dealing with challenging situations. I have the ability to form positive relationships with colleagues and I believe that team work is great key to performance. I always deliver quality, detailed, original (0% plagirism), well-researched and critically analyzed papers.
4.90+
1504+ Reviews
2898+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Write a Bronsted acid-base reaction in which act as conjugate acid - base pairs. H0-H andCHOH/CH,:
-
Q1: Edward is employed as an engine driver on a tourist steam train. For the year ended 30 June 2020, he has incurred the following expenditures: 1. Watch on a chain ($225): the watch helps Edward...
-
Identify the BrnstedLowry acidbase pairs in each of thefollowing equations: A.H3PO4( a q )+H2O( l )???H3O+( a q )+H2PO4?( a q ) acid H3PO4 conjugate acid H2PO4? ;base H2O conjugate base H3O+ base...
-
Firebird Bicycle Company, a fictional company, produces three brands of bicycles: Racing, Mountain, and Cruiser bicycles. The master schedule in the following table shows the demand requirements for...
-
1. After the Tarasoff case, do people generally have a duty to come to the aid of someone in danger? 2. What is the exception that this decision creates?
-
A number of salts containing the tetrahedral polyatomic anion, BF4- , are ionic liquids, whereas salts containing the somewhat larger tetrahedral ion SO42- do not form ionic liquids? Explain this...
-
1. Exhibit 12.13 presents free cash flow and economic-profit forecasts for ApparelCo, a $250 million company that produces mens clothing. ApparelCo is expected to grow revenues, operating profits,...
-
Lindstrom Design Services billed its customers a total of $245,100 for the month of August, including 9 percent federal excise tax and 5 percent sales tax. 1. Determine the proper amount of service...
-
Balance Sheet Preparation. Given the following account information for Denim Corporation, prepare a balance sheet in REPORT FORM for the company as of December 31,2020. All accounts have normal...
-
Work Study Problem 3.5 for the reverse of each reaction 3.16ac. CH3 H3C-C Br: I CH3 A CH3 HC-C+ B CH, H CH3 H3C-C-O -H I CH3 E H :0-H D F CH3 HC-C+ B T CH3 :Br C CH, H H3C-C-0-H CH3 E CH, H I OH...
-
In the following reactions, label the conjugate acidbase pairs and specify within each pair which is the acid and which is the base. Then draw the curved-arrow notation for these reactions in the...
-
A student has 65-cm-long arms. What is the minimum angular velocity (in rpm) for swinging a bucket of water in a vertical circle without spilling any? The distance from the handle to the bottom of...
-
1. List at least three (3) ways a business can anticipate potential problems to prevent complaints. 2. Explain how to identify customer needs and expectations. 3. List at least five (5) ways to build...
-
you have taken over a company with 4 employees and you have 1 millon dollars with you.as business management student,you are expected to take 5 business decisions ensuring that the company is able to...
-
Draw the Diamond - E ( SERVO ) model of strategic management and provide ONE WORD ( or short phrase ) that best describes the relationships between the elements of the model. ( up to 1 0 points )
-
If a patient's X-ray is rejected (at the end of the 22-minute evaluation by the doctor), she has a second X-ray taken (assume that the second X-ray will always be accepted) and this new X-ray must be...
-
What are the cognitive appraisal processes involved in stress perception, and how can cognitive-behavioral techniques such as cognitive restructuring and mindfulness-based interventions help...
-
Describe the functions of water and oxygen in the human body.
-
How do individual companies respond to economic forces throughout the globe? One way to explore this is to see how well rates of return for stock of individual companies can be explained by stock...
-
Predict the product(s) of each of the following biological reactions by interpreting the flow of electrons as indicated by the curvedarrows: (b) H3C (OPo,- (a) R. OPP (c) :Base 2-0? H C %24
-
Reaction of 2-methylpropene with HBr might, in principle, lead to a mixture of two alkyl bromide addition products. Name them, and draw their structures.
-
Draw the structures of the two carbocation intermediates that might form during the reaction of 2-methylpropene with HBr (Problem 5.40). Well see that the stability of carbocation depends on the...
-
Provide a graph chart or data with sample numbers indicating Valuing Stocks and Bonds?
-
I just need help with part b. It says that the answer is not complete and some are wrong. So can you kindly fix it for me and give me the full answers as it says the answer is "not complete". Thank...
-
What is Coke's average ownership percentage in its equity method investments? Goodwill is 7000 Calculate the firm's current ratio (current assets/current liabilities). Calculate the current ratio...

Study smarter with the SolutionInn App


