In the following reactions, label the conjugate acidbase pairs and specify within each pair which is the
Question:
In the following reactions, label the conjugate acid–base pairs and specify within each pair which is the acid and which is the base. Then draw the curved-arrow notation for these reactions in the left-to-right direction.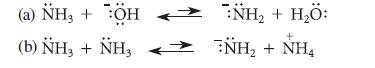
Transcribed Image Text:
(a) NH3 + :ÖH (b) NH3 + NH3 :NH, + H,Ö: + :NH, + NH,
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (4 reviews)
a HNH b H conjugate basea...View the full answer

Answered By

JAPHETH KOGEI
Hi there. I'm here to assist you to score the highest marks on your assignments and homework. My areas of specialisation are:
Auditing, Financial Accounting, Macroeconomics, Monetary-economics, Business-administration, Advanced-accounting, Corporate Finance, Professional-accounting-ethics, Corporate governance, Financial-risk-analysis, Financial-budgeting, Corporate-social-responsibility, Statistics, Business management, logic, Critical thinking,
So, I look forward to helping you solve your academic problem.
I enjoy teaching and tutoring university and high school students. During my free time, I also read books on motivation, leadership, comedy, emotional intelligence, critical thinking, nature, human nature, innovation, persuasion, performance, negotiations, goals, power, time management, wealth, debates, sales, and finance. Additionally, I am a panellist on an FM radio program on Sunday mornings where we discuss current affairs.
I travel three times a year either to the USA, Europe and around Africa.
As a university student in the USA, I enjoyed interacting with people from different cultures and ethnic groups. Together with friends, we travelled widely in the USA and in Europe (UK, France, Denmark, Germany, Turkey, etc).
So, I look forward to tutoring you. I believe that it will be exciting to meet them.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
In the following acid-base reactions, 1. Determine which species are acting as electrophiles (acids) and which are acting as nucleophiles (bases). 2. Use the curved-arrow formalism to show the...
-
A music performer, DJ DD walked offstage and refused to continue his show after at least one member of the audience started spitting at him and throwing drinks. Do you think that DJ DD's refusal to...
-
Question 1 This question is a long free-response question. Show your work for each part of the question. ? A cylindrical object can roll down an incline, as shown in Figure 1. The incline is slightly...
-
Explain the economic basis for the U shape of the long-run average total cost curve.
-
Is the bystander rule immoral? What are the differences between law and morality?
-
The boiling points, surface tensions, and viscosities of water and several alchohols are as follows: (a) For ethanol, propanol, and n-butanol the boiling points, surface tensions, and viscosities all...
-
2. Since growth is stable for ApparelCo, you decide to start the continuing value with year 3 cash flows (i.e., cash flows in year 3 and beyond are part of the continuing value). Using the key value...
-
In a recent year, Coach, Inc, a designer and marketer of handbags and other accessories, issued 12,100 shares of its $0.01 par value stock for $344,000 (these numbers are rounded). These additional...
-
answer both please Question 7 Not yet answered Which of the following statements most likely describes a situation that would motivate a manager to issue low quality financial reports? Points out of...
-
Write a Brnsted acidbase reaction in which act as conjugate acidbase pairs. H/-:H and CH,H/CH,:-
-
A histidine residue (B), one of the functional groups in the structure of a certain enzyme, has a conjugate-acid pK a = 7.8. What is the fraction of each form (BH and B) present at physiological pH...
-
Use the procedure described in Lemma 1.60 to convert the following finite automata to regular expressions. a a 1 ,b 1 b a b a 3 () (b)
-
Hardwick Corporation manufactures fine furniture for residential and industrial use. The demand for the company's products has increased tremendously in the past three years. As a result, the company...
-
Problem 3: Use the product rule to find the following derivatives. Leave your answer in the form f'(x)g(x)+ f (x) g' (r). That is, do not simplify. (a) s(t)=t3 cos (t) (b) F(y): = (12-1) (v + 5 y)...
-
Do an internet search of two or three organizations in your field of study (Human Resources). Review the organization or business and its hiring practices using some of the questions from the...
-
4. A process was set to meet the design specifications of USL = 26 and LSL = 18. The standard deviation of the process was found to be 1.2. The process mean was set to 22.5. a) Calculate the process...
-
Evaluate the broad environment, e.g., political, social, legal, in which the industry of OCSIP is located. How does this affect the industry?
-
Explain pH and how to use the pH scale.
-
Provide a few individual examples who revealed what aspects of emotional intelligence?
-
Name the following aldehydes and ketones according to IUPAC rules: (c) (a) , (b) CCH2 CHCH2CHCH C CHCCH2CH2H2H-CH (e) CH (f) (d) "op CH;CH3CHCH,C,H , * " "CH
-
Draw structures corresponding to the following names: (a) 3-Methylbutanal (b) 4-Chloro-2-Pentanone (c) Phenyl acetaldehyde (d) cis-3-tert-Butylcyclohexanecarbaldehyde (e) 3-Methyl-3-butenal (f)...
-
How would you prepare pentanal from the following starting materials? (a) CH 3 CH 2 CH 2 CH 2 CH 2 OH (b) CH 3 CH 2 CH 2 CH 2 CH = CH 2 (c) CH 3 CH 2 CH 2 CH 2 CO 2 CH 3
-
Ventaz Corp manufactures small windows for back yard sheds. Historically, its demand has ranged from 30 to 50 windows per day with an average of 4646. Alex is one of the production workers and he...
-
Which of the following statements is not true regarding the $500 credit for dependent other than a qualifying child credit. Cannot be claimed on the same tax return if the child tax credit is also...
-
Grind Co. is considering replacing an existing machine. The new machine is expected to reduce labor costs by $127,000 per year for 5 years. Depreciation on the new machine is $57,000 compared with...

Study smarter with the SolutionInn App


