Write an equation for each of the following equilibria, and use Table 3.1 to identify the pK
Question:
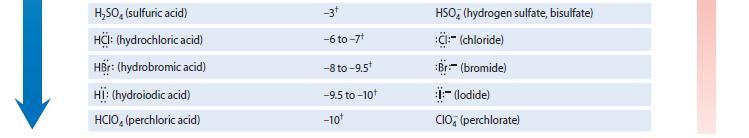
Write an equation for each of the following equilibria, and use Table 3.1 to identify the pKa value associated with the acidic species in each equilibrium.
(a) Ammonia acting as a base toward the acid water
(b) Ammonia acting as an acid toward the base water Which of these reactions has the larger Keq and therefore is more important in an aqueous solution of ammonia?
Transcribed Image Text:
GREATER ACIDITY TABLE 3.1 Relative Strengths of Some Acids and Bases Conjugate acid NH, (ammonia) RÖH (alcohol) HÖH (water) HPO (hydrogen phosphate) RSH (thiol) R,NH (trialkylammonium ion) NH, (ammonium ion) HCN (hydrocyanic acid) H₂PO, (dihydrogen phosphate) HSH (hydrosulfuric acid) R-C-OH (carboxylic acid) HE: (hydrofluoric acid) H₂PO (phosphoric acid) HNO, (nitric acid) H₂O* (hydronium ion) H₂C- -SO₂H (p-toluene- sulfonic acid) pK₂ ~35t 15-19 15.7 12.3 10-12* 9-11* 9.25 9.40 7.21 7.0 4-5* 3.2 2.2 -1.3 -1.7 -2.8+ Conjugate base -NH₂ (amide) RÖ:- (alkoxide) HÖ:- (hydroxide) PO (phosphate) RS (thiolate) R₂N: (trialkylamine) H₂N: (ammonia) -:CN (cyanide) HPO (hydrogen phosphate) HS:- (hydrosulfide) R-C-Ö: (carboxylate) F:-(fluoride) H₂PO (dihydrogen phosphate) NO; (nitrate) H₂O (water) H₂C- (p-toluene- -SO sulfonate, or "tosylate") GREATER BASI CITY
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
a Ammonia acting as a ba...View the full answer

Answered By

JAPHETH KOGEI
Hi there. I'm here to assist you to score the highest marks on your assignments and homework. My areas of specialisation are:
Auditing, Financial Accounting, Macroeconomics, Monetary-economics, Business-administration, Advanced-accounting, Corporate Finance, Professional-accounting-ethics, Corporate governance, Financial-risk-analysis, Financial-budgeting, Corporate-social-responsibility, Statistics, Business management, logic, Critical thinking,
So, I look forward to helping you solve your academic problem.
I enjoy teaching and tutoring university and high school students. During my free time, I also read books on motivation, leadership, comedy, emotional intelligence, critical thinking, nature, human nature, innovation, persuasion, performance, negotiations, goals, power, time management, wealth, debates, sales, and finance. Additionally, I am a panellist on an FM radio program on Sunday mornings where we discuss current affairs.
I travel three times a year either to the USA, Europe and around Africa.
As a university student in the USA, I enjoyed interacting with people from different cultures and ethnic groups. Together with friends, we travelled widely in the USA and in Europe (UK, France, Denmark, Germany, Turkey, etc).
So, I look forward to tutoring you. I believe that it will be exciting to meet them.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Write an equation for each of the following reactions: a. 2-methyl-2-butanol + HCl b. 3-pentanol + Na c. Cyclohexanol + PBr3 d. 2-phenylethanol + SOCl2 e. 1-methylcyclopentanol + H2SO4, heat f....
-
Write an equation for each of the following reactions. If no reaction occurs, say so. a. methyl propyl ether + excess HBr (hot) - b. dibutyl ether + boiling aqueous NaOH - c. ethyl ether + cold...
-
Write an equation for each of the following reactions: a. 2-butene + HCl b. 3-hexene + HI c. 4-methylcyclopentene + HBr
-
A nutritionally defective E. coli strain grows only on a medium containing thymine, whereas another nutritionally defective strain grows only on a medium containing leucine. When these two strains...
-
Why might critics think it is dangerous to permit the FAA to perform both functions?
-
On a single plot, qualitatively sketch the distribution of molecular speeds for (a) Kr (g) at - 50oC, (b) Kr (g) at - 0oC, (c) Ar (g) at - 0oC
-
2. Exhibit 11.16 presents the income statement and balance sheet for PartsCo, a $1.2 billion supplier of machinery parts. The company is expected to increase revenues by 8 percent annually for the...
-
Webb Corporation prepares financial statements in accordance with IFRS. Selected accounts included in the property, plant, and equipment section of the company's statement of financial position at...
-
Suppose you buy one SPX call option contract with a strike of 1,300. At maturity, the S&P 500 Index is at 1,321. What is your net gain or loss if the premium you paid was $14?
-
The basicities of conjugate bases A increase with increasing pK a of the conjugate acids AH. How do the basicities of conjugate bases A change with increasing pK b ?
-
Using the pK a values in Table 3.1, calculate the equilibrium constant for each of the following reactions. (a) NH 3 acting as a base toward the acid HCN (b) F acting as a base toward the acid HCN...
-
On January 1, 2021, Tru Fashions Corporation awarded restricted stock units (RSUs) representing 12 million of its $1 par common shares to key personnel, subject to forfeiture if employment is...
-
Manufacturing company produces $3800 worth of products weekly. If the cost of raw materials to make this product is $400, and the labour cost is $360, calculate the productivity.
-
1-You are a very well-recognized professional in your area, with many years of experience solving international conflicts. There is a company in the middle of two European countries that are fighting...
-
Find the solution u = u(x,y) of the following problem on the set R. u du - 4, (1.4) Ju(0,y) =3y, u(x, 0) = 0. (1.5) ay
-
Scenario A Sports Club 10 Highfield Sports Club has organised a fundraising event. 300 tickets have been sold at a price of $2.50 each. Money taken at the event Percentage of money (E) taken (96)...
-
Shamrock Investments has three divisions (Green, Clover, Seamrog) organized for performance evaluation purposes as investment centers. Each division's required rate of return for purposes of...
-
Describe how the change in shape of a protein may be either abnormal or associated with normal function.
-
Subprime loans have higher loss rates than many other types of loans. Explain why lenders offer subprime loans. Describe the characteristics of the typical borrower in a subprime consumer loan.
-
Draw an energy diagram for a two-step exergonic reaction whose second step is faster than its first step.
-
Draw an energy diagram for a reaction with Keq = 1. What is the value of G in this reaction?
-
The addition of water to ethylene to yield ethanol has the following thermodynamic parameters: (a) Is the reaction exothermic or endothermic? (b) Is the reaction favorable (spontaneous) or...
-
Docs Auto Body has budgeted the costs of the following repair time and parts activities for 2009: Doc's budgets 6,000 hours of repair time in 2009. A profit margin of $7 per labour hour will be added...
-
QUESTION 28 In a perpetual inventory system, the cost of inventory sold is: Debited to accounts receivable. Debited to cost of goods sold. O Not recorded at the time goods are sold. O Credited to...
-
The following financial statements and additional information are reported. IKIBAN INC. Comparative Balance Sheets June 30, 2019 and 2018 2019 2018 $105,709 69,500 66,800 4,700 246,700 127,eee...

Study smarter with the SolutionInn App


